General characteristics.
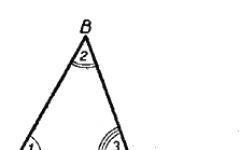
The term transition element is usually used to refer to any element with d or f valence electrons. These elements occupy a transitional position in the periodic table between electropositive s-elements and electronegative p-elements (see § 2, 3).
d-elements are usually called main transition elements. Their atoms are characterized by the internal structure of d-subshells. The fact is that the s-orbital of their outer shell is usually filled before the filling of the d-orbitals in the previous electron shell begins. This means that each new electron added to electron shell the next d-element, in accordance with the principle of filling (see § 2), falls not on the outer shell, but on the inner subshell preceding it. Chemical properties of these elements are determined by the participation of electrons from both of these shells in reactions.
d-Elements form three transition series - in the 4th, 5th and 6th periods, respectively. The first transition series includes 10 elements, from scandium to zinc. It is characterized by the internal configuration of -orbitals (Table 15.1). The orbital is filled earlier than the orbital because it has lower energy (see Klechkovsky's rule, § 2).
It should be noted, however, that there are two anomalies. Chromium and copper have only one electron in their -orbitals. The fact is that half-filled or filled subshells are more stable than partially filled subshells.
The chromium atom has one electron in each of the five -orbitals that form the -subshell. This subshell is half-filled. In a copper atom, each of the five -orbitals contains a pair of electrons. A similar anomaly is observed in silver.
As a manuscript
PHASE EQUILIBRIA IN NITROGEN - ALUMINUM - TRANSITION METAL SYSTEMS IV - V GROUPS.
01.04.07 - Physics of condensed matter
Moscow 2004
The work was completed at the department general chemistry Faculty of Chemistry Moscow State University them. M.V. Lomonosov and at the Institute of Metallurgy and Physics of Metals named after. G.V. Kurdyumov Central Research Institute of Chermet named after. I.P. Bardina.
Scientific director
Doctor of Physical and Mathematical Sciences, Professor A.I. Zaitsev Scientific consultant
candidate chemical sciences, senior researcher Kalmykov K.B. Official opponents:
doctor technical sciences, Professor Kraposhin B.S.
Doctor of Physical and Mathematical Sciences, Professor Kaloshkin S. D.
Lead organization:
Institute of Metallurgy and Materials Science named after. A.A. Baykova
The defense of the dissertation will take place on November 12, 2004 at D o’clock at a meeting of the dissertation council D 141.04.02 FSUE TsNIIchermet im. I.P. Bardin at the address: 105005, Moscow, st. 2nd Baumanskaya; 9/23.
The dissertation can be found in the technical library of the Central Research Institute of Chermet named after. I.P. Bardina.
Phone for inquiries: 777-93-50
Scientific Secretary
dissertation council D 141.04.02, candidate of technical sciences,
senior researcher ¿^G^sä^A-^ Aleksandrova N. M.
GENERAL DESCRIPTION OF WORK.
RELEVANCE OF THE TOPIC: Compositions based on complex nitrides of aluminum and transition metals of groups IV - V are increasingly used in various branches of industry and technology. They are the basis for creating wear-resistant and protective coatings, diffusion barriers in microelectronics, high-temperature metal-ceramic, composite materials, ceramics, etc. No less important role compounds of A1 and elements of groups IV - V with nitrogen play a role in the design and production of a wide range of grades of steels and alloys, especially with a high nitrogen content. Naturally, the physical, physicochemical and mechanical properties of the listed materials are directly related to the type and quantities of nitrogen-containing phases formed. Accurate data on the composition and conditions of existence of complex compounds are also of fundamental theoretical importance for understanding the nature chemical bond and other key characteristics that determine the degree of their sustainability. To predict the synthesis conditions and stability of nitrides, reliable information about phase equilibria is required. Constructing multicomponent phase diagrams involving nitrogen is very challenging. simple task due to the low thermodynamic incentives for the formation of mixed compounds from double phases adjacent in the state diagram, low rates of diffusion of components in them, as well as the complexity and low accuracy of determining the true nitrogen content. Therefore, the currently available information is fragmentary and extremely contradictory both regarding the composition of ternary nshrids and the position of phase equilibrium lines. It is mainly obtained by annealing powder compacts, in which it is difficult to achieve an equilibrium state of the alloy.
OBJECTIVE OF THE WORK: Development of a new approach to the study of phase diagrams of multicomponent nitride systems, based on the use of a complex of modern experimental techniques of physicochemical analysis, methods of thermodynamic analysis and calculation, which makes it possible to determine with high accuracy the conditions for the coexistence of phases and obtain comprehensive evidence of their compliance with equilibrium. Study phase equilibria in the solid-phase region of ternary systems aluminum - nitrogen - metal of 1U-U groups at a temperature of 1273 K. SCIENTIFIC NOVELTY:
Methods of thermodynamic analysis and calculations have been used to show the inconsistency of the available experimental data on the conditions of phase equilibrium in the T1-A1-N and T1-A1-M systems;
Thermodynamic modeling, analysis and calculation of phase equilibria in the &-A1-N and Sh-A1-K systems were carried out. Found for the first time
thermodynamic functions of ternary compounds formed in these systems;
The solid-phase regions of the phase diagrams of the Ti-Al-N, Zr-Al-N and Hf-Al-N systems at 1273 K were plotted;
The nature of phase equilibria in the Nb-Al-N system at a temperature of 1273 K has been established. SCIENTIFIC AND PRACTICAL SIGNIFICANCE OF THE WORK:
The information obtained about the equilibrium conditions and thermodynamic functions of phases in M-A1-N systems (hereinafter. M = Ti, Zr, Hf, Nb) is a fundamental scientific basis for the development of coatings, ceramic and metal-ceramic, composite materials important for microelectronics , energy, mechanical engineering. They make it possible to determine technological parameters for the production and processing of such materials, and are also of fundamental importance for predicting the phase composition and properties of a wide range of steels and alloys with a high nitrogen content. RELIABILITY AND VALIDITY:
Data obtained by various methods of physicochemical analysis on samples of alloys synthesized by various methods (nitriding of binary alloys, long-term homogenizing annealing, diffusion pairs), using modern experimental approaches and equipment, such as electron probe microanalysis, scanning electron microscopy, X-ray phase analysis, in all cases were in excellent agreement both with each other and with the results of thermodynamic calculations.
2. Structure of the solid-phase region of the isothermal section of the Ti-Al-N phase diagram at a temperature of 1273 K.
3. Results of thermodynamic analysis and calculation of phase equilibria in the Zr-Al-N system at 1273 and 1573 K.
4. Structure of solid-phase regions of state diagrams of Zr-Al-N, Hf-Al-N, Nb-Al-N systems at 1273 K.
APPROBATION OF WORK AND PUBLICATION. The main results of the work were presented at: the International Conference “VIII International conference of crystal chemistry of intermetallic compounds” (Lviv, Ukraine, 2002); International Conference of Undergraduate and Postgraduate Students in Basic Sciences “Lomonosov-2003”, (Moscow, 2003); International conference "Theory and practice of technologies for the production of products from composite materials and new metal alloys (T11KMM)", (Moscow, Moscow State University, 2001, 2003). Based on the dissertation materials, 4 articles were published. SCOPE AND STRUCTURE OF THE DISSERTATION. The dissertation consists of an introduction, a literature review, an experimental part, a discussion of the results,
conclusions and a list of references in the amount of 204 titles. The work is presented on 138 pages of typewritten text, including 70 figures and 26 tables.
The second part examines the patterns of interaction of nitrogen with elements IV-V groups, provides information about physical and chemical properties and methods of nitride synthesis. It is shown that double diagrams states M-N have not been fully studied. Only the existence of MN and M2N nitride phases has been reliably established, while the formation of other nitride phases is questionable due to possible stabilization by oxygen.
The main part of the literature review is devoted to the analysis of information about the structure of the M-A1-N phase diagrams. The M-A1-N phase diagrams have been studied to a much lesser extent than binary alloys. Data on the conditions of phase equilibrium in the Zr-Al-N, Hf-Al-N and Nb-Al-N systems are currently practically absent. Information about the phase diagram of the Ti-Al-N system contains a number of fundamental contradictions. EXPERIMENTAL PART. §1. Sample preparation procedure.
The starting materials used were Ti, Zr, Hf-iodide and in the form of powders with a purity of 99.5%, Nb - sheet vacuum melting with a purity of 99.99% and powder with a purity of 99.5%, nitrogen GOST 9293-74 OSCH (99.996 vol. % N2) 02< 0,001 об.%, mass fraction water vapor< 0,005 %). Порошки HfN, ZrN и AIN - марки «Ч», пластины AIN, полученные методом спекания с добавками У2О3.
Double M-A1 alloys were produced by fusing samples of components in a LAYBOLD HERAUES arc furnace with a non-consumable tungsten electrode in an atmosphere of purified argon. To increase the homogeneity of the ingots, they were remelted five times. The synthesized samples were wrapped in niobium foil and subjected to homogenizing annealing at 1273 K (100 hours) in evacuated quartz ampoules in electrical resistance furnaces, followed by quenching in water. The compositions of the alloys, their phase composition and homogeneity were controlled by electron probe microanalysis using a CAMEBAX-microbeam device (Table 1). §2. Methodology for studying samples.
The following research methods were used in the work:
Electron probe microanalysis using the CAMEBAX-microbeam device at accelerating voltages of 15 and 30 kV; preliminary analysis for impurities was carried out on a KEVEX energy-dispersive analyzer.
Scanning electron microscopy using JEOL and CAMEBAX-microbeam devices; The image was obtained in secondary electrons at accelerating voltages of 15 and 20 kV. The resulting images were processed and the phase relationship in the samples under study was determined.
Optical microscopy", using the methods of dark field, bright field, polarized light, differential interference contrast according to Nomarski. Studies were carried out on the "UEYA8AMET-2" device using a magnification ><300 и х400.
X-ray phase analysis using the powder method was carried out on DRON-4 and 8TAB1-R diffractometers from Yashe (CuK, CoK radiation).
Table 1.
Chemical and phase composition of binary alloys of M-A1 systems.
No. Composition (EPMA), at.% Phase composition No. Composition (EPMA), at.% Phase composition
System I - A1
1 25.6 74.4 t13, T1A12 4 69.6 30.1 T13A1
2 38.3 61.7 Т1А12, Т1А1 5 77.1 22.9 Ть,А1
h 54.9 45.1 T1A1, T13A1 6 89.1 10.9 "SP)
System Xg - A1
1 28.5 71.5 gA13, bgMg 5 60.1 39.9 Kht'RAb Tg2M
2 33.3 66.7 bxk\g 6 65.8 34.2
3 47.5 52.5 2g2A13, 2GA1 7 76.7 23.3 7Х2А\,
4 58.3 41.7 Хт4А1ъ ЪсгА\г
System Sh - A1
1 31.7 68.3 N£A13, ША12 4 53.8 46.2 NSh, N£(A13
2 36.8 63.2 NSh2, ShchA13 5 62.4 [ 37.6 Sh3A12, Zh5A13
3 43.2 56.8 NG2A13, NSh 6 77.8 | 22.2 Yu2A1, a(H0
System No. - A1
1 37.8 62.2 LbAb, Nb2A1 4 71.3 28.7 Mb2A1, N>3A1
2 51.2 48.8 1МА13, Мь2А1 5 82.8 17.2 №>3А1, а(№>)
3 63.5 36.5 Lb2A1
§ 3. Development of a methodology for studying phase diagrams involving nitrogen.
To study phase equilibria in ternary systems M-A1-N we used the complex modern methods physical and chemical analysis, which included: nitriding of powders of binary M-A1 alloys in a nitrogen atmosphere, diffusion couples and long-term homogenizing annealing of the alloys.
For nitriding, powders of M-A1 binary alloys were placed in A1203 crucibles and subjected to isothermal exposure in a thermocompression annealing installation original design in a nitrogen atmosphere at a pressure of 5 MPa, a temperature of 1273 K for 1, 4, 9 and 16 hours. The phase composition of the samples was studied by X-ray phase analysis after each annealing.
To determine the influence of the duration of nitriding on the change in the composition of double nitride phases within the homogeneity region, we studied the dependence of the lattice parameter of zirconium and hafnium nitrides on
annealing time in a nitrogen atmosphere at a temperature of 1273 K and a pressure of 5 MPa. The lattice parameters of ZrN and HfN did not change during annealing for 4 and 13 hours, which indicates that in the systems under study, the duration of high-temperature nitriding has virtually no effect on the composition of the resulting nitride.
Diffusion pairs were prepared according to the “sandwich” type M/A1N/M in two ways: diffusion welding and surfacing. Diffusion welding was carried out in vacuum on a DSVU installation at temperatures of 1273 K for titanium, 1373 K for zirconium and niobium, and 1433 K for hafnium. The welding pressure was 17-20 MPa. Surfacing of Ti, Zr, Hf or Nb onto a 2x4x4 mm AIN plate was carried out in an electric arc furnace in an atmosphere of purified argon. The resulting pairs were annealed in evacuated quartz ampoules for 100 and 670 hours, and the structure of the resulting transition zones was studied using electron probe microanalysis, optical and scanning electron microscopy. The use of two methods for obtaining diffusion pairs excluded the possibility of the influence of physicochemical processes occurring at the interfaces when combining dissimilar materials into a single composition on the structure of diffusion zones and the nature of the results obtained.
To carry out studies of the third type, samples of two types were synthesized:
1) Mixtures of a certain composition were prepared from Zr, Hf, Nb and AIN powders. The mixtures were compressed at room temperature and pressure 10 MPa. The tablets were melted in an electric arc furnace in an argon atmosphere and subjected to long-term homogenizing annealing at 1273 K in evacuated quartz ampoules for 200 and 670 hours to achieve an equilibrium phase configuration.
2) A1N plates were wrapped in titanium or niobium foil and then melted in an electric arc furnace. Then the samples were subjected to long-term annealing according to the described procedure. The criterion for achieving an equilibrium state was the constancy of the type and number of phases with increasing annealing duration.
Calculation and analysis of phase equilibria in the systems under study were carried out in accordance with the fundamental laws of thermodynamics. When analyzing each specific composition, all possible combinations of phases were considered, a combination of which it could be represented. The phase combination corresponding to the minimum Gibbs energy of the system was considered to correspond to stable equilibrium, and its characteristics (the nature and number of coexisting phases) were used in constructing the phase diagram. All other combinations of phases were considered metastable and their characteristics were not taken into account. To reduce the thermodynamic functions to the same standard states of the components, we used the available information about their stability parameters or the Gibbs energy of phase transitions. The calculation algorithm was implemented in the form of a special computer program, which involved repeated procedures for determining the phase composition of the system for a variety of
points covering the entire range of compositions in the space of component concentrations at a given temperature.
Preliminary experiments and calculations made it possible to formulate the principles for choosing the compositions of the samples under study, the modes of their nitriding and heat treatment, which make it possible to achieve the same state of the alloy in different ways and obtain comprehensive evidence of its compliance with equilibrium. RESULTS AND DISCUSSION. § 1. Phase equilibria in the T1-A1-1Ch system.
The results of preliminary experiments showed that the most effective method The study of phase equilibria in the T!-Al-N system is the nitriding of powdered samples from the gas phase. Table 2 presents the results of X-ray phase analysis of samples after annealing in a nitrogen atmosphere at 1273 K for 1 hour. In the first five alloys, the T12AM ternary compound is formed. The results obtained indicate the existence of the following phase fields in the Tb-Al-M system: TlA1s-TlA1K-AS, TgAM-AM-"Sh, TShs-T^A^-IgASh and T-TSh-oOP).
Table 2.
Phase composition of powdered samples of the T1-A1-N system before and after annealing in a nitrogen atmosphere at T = 1273 K, p(N2) = 5 MPa.
Alloy No. Phase composition
before nitriding after nitriding
1 TiAl3, TiAl2 Ti2AlN, TiAl3, A1N
2 TiAl2, TiAl Ti2AlN, TiAl3, TiAl2
3 TiAl, T13AI Ti2AlN, TiNi.x, A1N
4 Ti3Al Ti2AlN, TiN,.x
5 Т1зА1 TijAIN, TiNi.x
6 a(Ti) TiNi.jb Ti2N, a(Ti)
To study the titanium-rich region of the phase diagram, the methods of diffusion pairs and long-term homogenizing annealing were used. In the diffusion zone of the A1N/Ti sample after 200 hours of isothermal exposure at T = 1273 K, the formation of two intermediate layers was recorded: a titanium nitride layer containing inclusions of the Ti3AlN ternary phase, and a layer of solid solution based on a(Ti) with an aluminum concentration of up to 19 at.% . Figure 1(a) shows the structure of an AlN/titanium interlayer sample with a thickness of 150 mkm/AIN. After 200 hours of annealing, a layer of titanium nitride with a thickness of about 30 μm is formed on the surface of aluminum nitride; the middle of the interlayer is a Ti3AlN phase with inclusions of titanium nitride TiN].x. The results obtained indicate the existence of AlN-TiNi.„TiN!.x-Ti3AlN, Ti3AlN-a(Ti) terminals.
For precise definition To determine the position of equilibrium lines in titanium-rich alloys with the participation of slowly forming complex nitride Ti3AlN, two samples were synthesized by fusing samples of titanium and aluminum nitride powder in a mole ratio of 3/1 and 2/1. The first alloy acquired a constant phase composition after 200 hours of annealing
TP^-x+"PsAP^+aSP). According to scanning electron microscopy and X-ray phase analysis (Fig. 1 b), in the second sample after 200 hours of annealing there were 4 phases: TO^." "PzAGY, a(Tl) and "PzA1.
Moreover, T13AM inclusions were found around titanium nitride particles, which indicates insufficient homogenization time. After 670 hours of annealing, the phase composition of the sample acquired a stable configuration: TOL-"PzASH+a(T0) (Fig. 2).
TIASH TAA1 -
Rice. 1. Microstructure of samples of the “L - A1 - >1” system:
a - AMGP/AM after annealing for 200 h, 1273 K, secondary е, xООО; b - A1K+2GP after annealing for 200 hours, 1273 K, secondary е, xООО.
n -^zASH A -0(14)
20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 20 Fig. 2. X-ray diffraction pattern of the AlN+2T1 sample after annealing for 670 h, 1273 K.
To determine the position of phase equilibrium lines at low nitrogen concentrations, thermodynamic calculations were used. The existence of a liquid solution based on aluminum and a- and 3-solid solutions based on titanium was not taken into account, since the melt lies outside the region of interest for solid-phase equilibria, and equilibria with solid solutions have been studied in detail experimentally. Currently, experimental data on the Gibbs energy of formation ( There are no A/7) phases "PzAPCh, T12A1N, T1A12. There are only estimates. Therefore, at the first stage, these unknown characteristics were found by indirect optimization method. The essence of the method was to select the A/? values of these compounds so that they satisfy the experimentally established conditions As a result, the following values were found: A/7(T13A1K) = -360.0 kJ/mol; D/7SP2A1M) = -323.3 kJ/mol; /mol. Subsequently, they were used to calculate phase equilibria in alloys, the experimental study of which is difficult or impossible. The constructed isothermal (T = 1273 K) section of the phase diagram of the P-ANCh system is presented in Fig. 3.
I - compositions of the initial binary alloys "P-A1. X - compositions of nitrided alloys, ♦ - compositions of ternary alloys T1 + A1KG, - - - ■ diffusion path. The results of thermodynamic calculations are highlighted in the background.
The results obtained are in some contradiction with existing data, schematically shown in Fig. 4. As can be seen, the authors found that AM is in equilibrium with "PAb, T1A12> T1A1, T12A1N and TO^.* (Fig. 4 a). Figure 4 (b) shows the results of thermodynamic analysis and calculation of phase equilibria performed in At work, aluminum nitride is in equilibrium only with PAb, T^AM and Tn^. This is in good agreement with the present results.
Rice. 4. Isothermal cross-section of the system at 1273 K:
a - according to data; b - according to data, trIZASH, gg-T^AM, 1-T1A1z, 2-T\tsA\i, 3-TSh2,4-T1A1, b-T^A).
The thermodynamic analysis of phase equilibria in the PA system carried out in this work made it possible to identify the causes of the noted contradictions. It turned out that the formation of ternary nitrides from the initial binary alloys in many cases is accompanied by an insignificant change in the Gibbs energy, amounting to only a few hundred J/mol. Therefore, The authors who used the method of annealing powder mixtures of binary compositions needed very long annealing time intervals to achieve an equilibrium state. This, apparently, was not always possible, on the contrary, the interaction of titanium-aluminum alloy powders with nitrogen used in the proposed work is accompanied by a significant (hundreds). kJ/mol) gain in the Gibbs energy, which allows you to quickly achieve an equilibrium state § 2. Conditions for equilibrium phases in the system r-Al-P*.
The study of phase equilibria in the g-AMCH system was carried out according to a similar scheme. Previously, thermodynamic modeling and calculation of phase equilibria in the system were carried out using available information about thermodynamic properties double phases (Table 3) and data on the phase diagram at 1273 and 1573 K (Fig. 5). The calculation makes it possible to completely reproduce experimental data on phase equilibria at 1573 K. On the other hand, information about the conditions of phase equilibrium at 1273 K cannot be reproduced by thermodynamic calculation.
In particular, the equilibrium A1Ni-2r3AM is realized only at the values (1/5)L/7(7r3A1M)< -92,0 кДж/моль. Однако, при этом устойчивой оказывается комбинация фаз АМ~гг3А1^-7гА12. Увеличение энергии Гиббса образования 7г3АГМ приводит к появлению трехфазного равновесия г^-АМ-ггА12.
Table 3.
Gibbs energy of formation of binary compounds of the system Bx - A1 - N from hcp-gg, fcc-A1 and N2(gas).
Phase D /J=a+bT, J/mol. Phase AfG=a+bT+cTlnT, J/mol.
(l/4)Zr3Al 36163 4.421 (l/2)ZrAl 64950 11.014 0
(l/3)Zr2Al 48358 6.492 (l/5)Zr2Al3 55323 27.830 4.329
(l/8)Zr5Al3 51484 5.749 (l/3)ZrAl2 51266 29.726 4.417
(l/5)Zr3Al2 55180 6.734 (l/4)ZrAl3 47381 24.373 3.854
(l/7)Zr4Al3 58480 8.236 (l/2)ZrN 181795 46.024 0
(l/9)Zr5Al4 55424 5.320 (1/2) AIN 163532 57.760 0
The established coexistence of the ArN-Zr3AlN-Zr2Al3 phases is not reproduced at any values of A//(Zr3AlN). In addition, to ensure AlN-Zr3AlN equilibrium, it is necessary to reduce (l/5)A/?(Zr3AIN) from -73.0 kJ/mol at 1573 K to -92.0 kJ/mol at 1273 K. The latter is unlikely, since can occur only at unrealistically low values of the entropy of formation of the analyzed compound A£(Zr3AlN) = -380.0 J/mol-K.
Thus, the data on the conditions of phase equilibrium in the Zr-Al-N system found in the work for different temperatures of 1573 and 1273 K are internally contradictory and require detailed experimental verification.
Annealing of alloys of the Zr-Al system in a nitrogen atmosphere at a pressure of 5 MPa for 1 hour led to the formation of zirconium nitride ZrN and zirconium aluminide ZrAl3, regardless of the composition of the initial sample. An exception was observed only for alloys No. 5-No. 7 (Table 4), the diffraction patterns of which contained peaks corresponding to the ZrÀl2 compound. The presented results indicate the possibility of the existence of a heterogeneous field AlN-ZrAl3-ZrN, which contradicts the results of thermodynamic calculations. According to thermodynamic analysis, equilibrium of the ZrAl3 and ZrN phases in Zr-Al-N alloys should not occur, both in the presence and absence of complex nitrides. Indeed, additional isothermal exposure of the samples in a nitrogen atmosphere for 4 hours led to a decrease in the intensity of the peaks corresponding to the ZrAl3 compound and the appearance of lines of the ZrAl2 phase in the diffraction patterns; longer annealing caused the disappearance of the lines of the ZrAl3 compound in the diffraction patterns.
The described phenomenon is of a kinetic nature. Zirconium reacts with nitrogen much more intensely than aluminum, so zirconium nitride and the ZrAl3 phase, which is maximally depleted in zirconium, are first formed in the samples. As the isothermal holding time increases, aluminum reacts with nitrogen to form aluminum nitride A1N. As a result, the phase
ChtA\3 transforms into ChtA\2, forming the equilibrium composition rAl2-ASh-7rN. Thus, the study of the interaction of powdered Zr-A\ alloys with nitrogen confirmed the adequacy of the thermodynamic calculation and indicates the existence of two key phase fields in the 2x-Al-Na AlN-2gAl-7gA12 and AlN-2rAl-2gA12 system.
Rice. 5. State diagram of the 2g-A1-1M system:
a - according to data, 1273 K; b - according to data, 1573 K; c - real calculation, 1273 K; g - real calculation, 1573 K.
X-ray diffraction and electron probe analysis of a sample obtained by fusing zirconium and aluminum nitride powders with a ratio of moles Xr/AN = 3/1 after homogenization for 670 hours at 1273 K showed the presence of phases: 7gM, 7.g5A13M1_x and 2g3A1>1, components stable configuration. The study of the structure of the transition zones of diffusion pairs AGN/gg/AS and AlM/7,g made it possible to reveal the existence of two more phase fields 2rH-2r3A1K-a(2r) and 2rK-r2A13-r5A13N1.x (Fig. 6).
Table 4.
Phase composition of powdered 2g-Al alloys before and after annealing in a nitrogen atmosphere at T = 1273 K, p0^2) = 5 MPa.
Alloy No. Phase composition
Before nitriding After nitriding
1 ZrAl3, ZrAl2 1h. ZrN, AIN, ZrAl3
4 hours ZrN, AIN, ZrAl3, ZrAl2
2 ZrAl2 1 part ZrN, ZrAlj
4 hours ZrN, ZrAl3, ZrAb
3 Zr2Al3, ZrAl ZrN, AIN, ZrAl3
4 Z14AI3, Zr3Al2 ZrN, AIN, ZrAl3
5 ZrjAlz, ZrzAl ZrN, ZrAI2, ZrAI3
6 ZrsAlî, Zr2Al ZrN, ZrAl2, ZrAl3
7 ZTÎAI, 3(Zr) ZrN, ZtA12, ZrAl3
Rice. 6. Structure of transition zones of diffusion containers AIN with Zr: a - AIN/Zr/A1N 200 hours, x 1500; b - A1N/Zr, 200 hours, x 2000.
Due to the high rates of interaction of zirconium with nitrogen, equilibria involving the ZrAl, Zt4A13, ZrAl2 and Zr2Al phases could not be determined experimentally. To establish them, thermodynamic calculations were used. At the first stage, the Gibbs energy of formation of ternary nitrides was found by indirect optimization method: (l/5)A/?(Zr3AlN) = -76.0 kJ/mol; (1/(9-x)) D/Z^^АУ^.*) = -63.0 kJ/mol. The obtained values were used to find unknown phase equilibrium conditions. The results obtained are shown in Fig. 7.
The constructed state diagram of the Zr-Al-N system at 1273 K is in conflict with the data for this temperature, however, it practically coincides with the results obtained for 1573 K. Apparently, the duration of annealing used was not enough to achieve the equilibrium state of the alloy at a lower temperature. temperature 1273 K.
аА1з 2хАИ ¿ГдА^
ggА1 4 ъъА\
Rice. 7. Phase diagram of the 2g-A1-N system, 1273 K. ■ - compositions of the initial binary alloys of the 2g-A1 system, o - compositions of nitrided alloys, □ - composition of the ternary alloy 2g + AM.
Diffusion paths in the system Bx - A1 - N at 1273K. aaaaa - sample (¿лЛы+ТхгаЦуТт 670 hours.
AM/AS sample 200 hours
Sample A1Y/gg 200 hours.
§ 3. Structure of the state diagram of the Hf-Al-N system.
A similar situation occurs for the Hf-AI-N system. In Fig. Figure 8 shows the structure of the phase diagram at 1273 K, obtained in this work together with the data.
Almost all phases of the Hf-Al binary system are in equilibrium with hafnium nitride HfN. This is due to the low Gibbs energy of HfN formation. The ternary compound Hf3AlN forms regions of three-phase equilibrium only with the phases Hf5Al3, HfN and a(Hf). The binary compounds Hf2Al and Hf3N2 are realized only in very limited compositional regions of the ternary system. Aluminum nitride is in equilibrium with HfAl3 and HfN. § 4. Phase equilibria in the Nb-Al-N system.
In Fig. Figure 9 shows the state diagram of the Nb-Al-N system (T=1273 K), constructed in this work. The results obtained practically coincide with the work data for a temperature of 1773 K, shown below. The only difference is that at 1273 K in the Nb-N system, niobium nitride NbN is stable, which is in equilibrium with aluminum nitride and the Nb2N-based phase. The compound N>4N3 is present only in a limited range of compositions of ternary alloys. The ternary compound Nb3Al2N is in equilibrium with the phases AIN, NbAl3, NbAl2 and Nt^N. The Nb3Al-based phase and the niobium-based solid solution form a three-phase region with niobium nitride Nb2N. CONCLUSION.
In conclusion, the main results of the work are summarized. It has been shown that at high nitrogen contents, the most promising method for studying the phase diagrams of three- and more-component nitride systems is the nitriding of powdered binary alloys. At low nitrogen concentrations, the most adequate results are obtained by the methods of diffusion pairs and long-term homogenizing annealing. The commonly used technique for annealing powder compacts requires long-term isothermal exposure and at temperatures below 1473 - 1573 K, in many cases, does not allow achieving an equilibrium state of the alloy.
Using a complex of modern methods of physical and chemical analysis, state diagrams of the Ti-Al-N, Zr-Al-N, Hf-Al-N and Nb-Al-N systems at 1273 K were constructed. An approach based on the implementation of different paths was used in the work to achieve the same final state of the alloy. Data found using different techniques, are in good agreement both with each other and with the results of thermodynamic calculations, and therefore can be recommended for predicting phase equilibria in these systems and compositions based on them.
A general pattern in the structure of the phase diagrams of the studied M - Al - N systems is a decrease in the number and stability of complex nitride phases as the difference between the thermodynamic stability of the double phases MN and A1N increases. Thus, predicting the possibility of obtaining three-component nitride phases, including in steels and alloys, can be carried out by comparing the values of the Gibbs energy of formation of A1N and MN.
Rice. 8 State diagram Ш-А1-М:
a - according to 1273 K data; b - according to 1673 K data; c - according to the data of this work ■ - compositions of the initial binary alloys of the H£-Al system. - compositions of nitrided alloys (1 hour). A - compositions of nitrided alloys (4 hours), o - composition of the ternary alloy NX + AM. -*- - diffusion paths in the Ш"-А1-К system at 1273 K.
Rice. 9. State diagram >1b-A1-K:
a - according to this work, 1273 K:
■ - compositions of the initial binary alloys of the Mb-A! system. - compositions of nitrided alloys □ - composition of the ternary alloy ZKL + ASH.
Diffusion paths in the Mb-Al-N system at 1273K.
b - according to data, 1773 K.
2. Using modern approaches thermodynamic calculation and modeling of phase equilibrium conditions, an analysis of existing data on state diagrams of M-A1-M systems was carried out. Their inconsistency has been revealed and ways of optimal experimental research have been determined.
3. Using a complex of modern methods of physicochemical analysis, the patterns of interaction of elements in 85 samples of binary and ternary alloys of the M-A1-1Ch systems were studied.
4. A solid-phase state diagram of the Ti-ANN system at 1273 K has been constructed. It has been established that aluminum nitride is in equilibrium with the phases T1A13, Tl2ASh and "PM". The ternary compound T13A1N forms three-phase regions with the phases T12AGM, T1A1, T13A1, a( T1) and T1^.*. Parameters determined
crystal lattices ternary phases Ti2AlN (a=2.986(9)Â, c=13.622(5)Á), Ti3AIN (a=4.1127(17)Â), and the Gibbs energy of their formation from modifications of elements stable at this temperature: -360 .0 kJ/mol and -323.3 kJ/mol, respectively.
5. Phase equilibria in crystalline Zr-A!--N alloys at 1273 K were studied. The position of all regions of three-phase equilibria was reliably established. Aluminum nitride is in equilibrium with the ZrAl3, ZrAl2 and ZrN phases. The ternary phase Zr3AlN forms three-phase equilibrium fields with the ZrN, Zr5Al3Ni.x phases and the a(Zr)-based solid solution. The lattice parameters of the complex nitride Zr3AlN are a=3.366(6)Â, è=l 1.472(10)Â, c=8.966(9)Â, Gibbs energy of formation Ap = -460.0 kJ/mol.
6. It has been established that in solid compositions of the Hf-Al-N system at 1273 K, almost all double phases of the Hf-Al system are in equilibrium with hafnium nitride HfN. The ternary compound Hf3AlN forms regions of three-phase equilibrium with the phases Hf5Al3, HfN and the solid solution based on a(Hf). The double phases Hf2Al and Hf3N2 occur only in limited compositional regions of the ternary system. Aluminum nitride is in equilibrium with HfAI3 and HfN.
7. For the first time, an isothermal T=1273 K cross section of the solid-phase part of the state diagram of the Nb-AI-N system was constructed. The ternary compound Nb3Al2N is in equilibrium with the phases AIN, NbAI3, NbAl2 and Nb2N. The Nb3Al-based phase and the niobium-based solid solution form a three-phase field with Nb2N. Niobium nitride NbN is in equilibrium with aluminum nitride and NbzN.
LIST OF REFERENCES CITED:
Schuster J.C., Bauer J. The Ternary System Titanium-Aluminum-Nitrogen. //J.
Solid State Chem. 1984. V.53. p 260-265.
Chen G., Sundman B. Thermodynamic Assessment of the Ti-Al-N System. //J.
Phase Equilibria. 1998.V.19. No. 2, p. 146-160.
Schuster J.C., Bauer J., Debuigne J. Investigation of Phase Equilibria Related to
Fusion Reactor Materials: l.The Ternary System Zr-Al-N. //J. Nucl. Mater. 1983.
V.116, p.131-135.
Schuster J.C., Bauer J. Investigation of Phase Equilibria Related to Fusion Reactor
Materials: P. The Ternary System Hf-Al-N. //J. Nucl. Mater. 1984. V.120, p. 133-136.
Determination of the phase composition of such materials showed the presence of only double nitride phases. However, recent, thorough studies of M - Al - N alloys (hereinafter M = Ti, Zr, Hf, Nb) have revealed the existence of complex nitrides: Ti3AlN, TÎ2A1N, Ti3Al2N2; Zr3AlN, ZrsAbNj.x; Hf3AlN, Hf5Al3N; Nb3Al2N. Their properties have been practically unstudied, although there is good reason to believe that they may be unique. This is evidenced by the fact that composite materials based on a combination of double nitrides A1 and M have the maximum level physical characteristics precisely in the areas of composition of the ternary phases. For example, the abrasive properties of Ti - Al - N ternary compounds are twice as high as those of corundum and even than those of tungsten carbide.
An equally important role is played by compounds of A1 and elements of groups IV - V with nitrogen in the design and production of a wide range of grades of steels and alloys, especially with a high nitrogen content. Naturally, the physical, physicochemical and mechanical properties of the listed materials are directly related to the type and quantities of nitrogen-containing phases formed. Accurate data on the composition and conditions of existence of complex compounds are also of fundamental theoretical importance for understanding the nature of the chemical bond and other key characteristics that determine the degree of their stability. To predict the synthesis conditions and stability of nitrides, reliable information about phase equilibria is required. Constructing multicomponent phase diagrams with the participation of nitrogen is a very difficult task due to the low thermodynamic incentives for the formation of mixed compounds from double phases adjacent in the phase diagram, the low diffusion rates of components in them, as well as the complexity and low accuracy of determining the true nitrogen content. Therefore, the currently available information is fragmentary and extremely contradictory both regarding the composition of ternary nitrides and the position of the phase equilibrium lines. It was mainly obtained by one group of researchers by annealing powder compacts, in which achieving an equilibrium state of the alloy is difficult.
GOAL OF THE WORK:
Development of a new approach to the study of phase diagrams of multicomponent nitride systems, based on the use of a complex of modern experimental techniques of physicochemical analysis, methods of thermodynamic analysis and calculation, which makes it possible to determine with high accuracy the conditions for the coexistence of phases and obtain comprehensive evidence of their compliance with equilibrium. Study of phase equilibria in the solid-phase region of ternary systems aluminum - nitrogen - metal of IV - V groups at a temperature of 1273 K.
SCIENTIFIC NOVELTY:
Methods of thermodynamic analysis and calculations have been used to show the inconsistency of the available experimental data on the conditions of phase equilibrium in T1-Al-Ligg-Al-K systems;
A methodology has been developed for studying the phase diagrams of nitride systems, which is based on a set of modern methods of physical and chemical analysis and the implementation of different ways to achieve the same final state of the alloy, which allows obtaining comprehensive evidence of compliance with its equilibrium;
Thermodynamic modeling, analysis and calculation of phase equilibria in the systems Bx - A1 - N and NG - A1 - N were carried out. The thermodynamic functions of ternary compounds formed in these systems were found for the first time;
The solid-phase regions of the state diagrams of the P - A1 - N systems are constructed.
A1-S and NG-A1-S at 1273 K; The nature of phase equilibria in the Lib - Al - N system at a temperature of 1273 K has been established.
SCIENTIFIC AND PRACTICAL SIGNIFICANCE OF THE WORK:
The information obtained about the equilibrium conditions and thermodynamic functions of phases in systems M - A1 - N (M = T1, bx, H £ bb) is a fundamental scientific basis for the development of coatings, ceramic and metal-ceramic, composite materials, important for microelectronics, energy, and mechanical engineering . They make it possible to determine technological parameters for the production and processing of such materials, and are also of fundamental importance for predicting the phase composition and properties of a wide range of steels and alloys with a high nitrogen content.
RELIABILITY AND VALIDITY:
Data obtained by various methods of physicochemical analysis on samples of alloys synthesized by various methods (nitriding of binary alloys, long-term homogenizing annealing, diffusion pairs), using modern experimental approaches and equipment, such as electron probe microanalysis, scanning electron microscopy, X-ray phase analysis, in all cases were in excellent agreement both with each other and with the results of thermodynamic calculations.
THE FOLLOWING PROVISIONS ARE MADE FOR DEFENSE:
1. A technique for constructing phase diagrams of multicomponent nitride systems, based on a combination of a set of modern methods of physical and chemical analysis with various ways to achieve the same equilibria, thermodynamic modeling and calculation of phase equilibria.
2. Structure of the solid-phase region of the isothermal section of the phase diagram “L - A1 - N at a temperature of 1273 K.
3. Results of thermodynamic analysis and calculation of phase equilibria in the Tl - A1 - N system at 1273 and 1573 K.
4. Structure of the solid-phase regions of the state diagrams of the systems Zg - A1 - N. NG- A1 - N. N1) - A1 - N at 1273 K.
II. LITERATURE REVIEW
Conclusion of the dissertation on the topic "Physics of Condensed Matter"
VI. conclusions.
1. A methodology has been developed for studying the state diagrams of multicomponent nitride systems, based on a combination of methods of nitriding of binary alloys, long-term homogenizing annealing of three-component compositions, diffusion pairs, thermodynamic calculations and modeling of phase equilibria. It allows you to implement different ways to achieve the same final state of the alloy and obtain comprehensive evidence of compliance with its equilibrium. It has been established that when studying areas of state diagrams with high nitrogen concentrations, the most reliable and informative method is the nitriding method of binary alloys. At low nitrogen concentrations, the best results are obtained by the diffusion pair method.
2. Using modern approaches of thermodynamic calculation and modeling of phase equilibrium conditions, an analysis of existing data on the state diagrams of M-A1-I systems was carried out. Their inconsistency has been revealed and ways of optimal experimental research have been determined.
3. Using a complex of modern methods of physicochemical analysis, the patterns of interaction of elements in 85 samples of binary and ternary alloys of the M-A1-N systems were studied.
4. A solid-phase state diagram of the T1-A1-K system at 1273 K has been constructed. It has been established that aluminum nitride is in equilibrium with the phases IA13, "PgASH and TO^.*. The ternary compound TS3AIA forms three-phase regions with the phases TSgASH, T1A1, T13A1, a(P) and The parameters of the crystal lattices of the ternary phases T12ASh (a=2.986(9)A, c=13.622(5)A), T13ASh (a=4.1127(17)A), and the Gibbs energy of their formation from modifications of elements stable at this temperature: -360.0 kJ/mol and -323.3 kJ/mol, respectively.
5. Phase equilibria in crystalline alloys at 1273 K were studied. The position of all regions of three-phase equilibria was reliably established. Aluminum nitride is in equilibrium with the 2gAl3, ZmA\2 and ZgN phases. The triple phase ggzANYA forms fields of three-phase equilibria with phases
ZrsAbNi.x and a(Zr)-based solid solution. The lattice parameters of the complex nitride Z^AIN are d=3.366(6)А, ¿»=11.472(10)В, c=8.966(9)В, Gibbs energy of formation А/3 = -380.0 kJ/mol.
6. It has been established that in solid compositions of the Hf-Al-N system at 1273 K, almost all double phases of the Hf-Al system are in equilibrium with hafnium nitride HfN. The ternary compound Hf^AlN forms regions of three-phase equilibrium with the HfsAh, HfN phases and the a(Hf)-based solid solution. Double phases Hf2Al, ^N2 occur only in limited compositional regions of the ternary system. Aluminum nitride is in equilibrium with HgAl3 and HfN.
7. For the first time, an isothermal T=1273 K section of the solid-phase part of the state diagram of the Nb-Al-N system was constructed. The ternary compound Nl^AhN is in equilibrium with the phases AIN, NbAb, NbAb and Nb2N. The Nb3Al-based phase and the niobium-based solid solution form a three-phase field with Nb2N. Niobium nitride NbN is in equilibrium with aluminum nitride and Nb2N.
V. CONCLUSION.
A general pattern in the structure of the phase diagrams of the studied M - Al - N systems is a decrease in the number and stability of complex nitride phases as the difference between the thermodynamic stability of the double phases MN and A1N increases, which is characterized by the Gibbs energy of formation Zl/7(A1N) = -180.0 kJ/mol, Zl/7(TiN)=-217.8 kJ/mol, 4G(ZrN)=-246.4 kJ/mol, ZlyG(HfN)-251.0 kJ/mol, zl/7(NbN) =-110.7 kJ/mol. Thus, in the systems Ti - Al - N and Zr - Al - N at 1273 K there are two complex nitrides TijAIN, Ti2AlN and Z^AIN, ZrsAbNi-x, respectively. Moreover, at high temperatures in Ti - Al - N alloys, the TÎ4A1N3.X phase is stable, and the ZrsAbNi-* compound cannot be considered ternary, since it is isostructural with the ZrsAb intermetallic compound. In the phase diagrams of Hf - Al - N and Nb - Al - N there is only one complex compound Hf3AlN and Nb3Al2N, respectively.
In the Ti - Al - N and Nb - Al - N systems, aluminum nitride is in equilibrium with the corresponding complex nitride, titanium or niobium nitrides and titanium or niobium aluminides with the maximum concentration of aluminum. In systems with zirconium and hafnium, the AIN - M3AIN equilibrium disappears. This is caused by an increase in the thermodynamic stability of the double nitride phases ZrN and HfN. Thus, predicting the possibility of obtaining three-component nitride phases, including in steels and alloys, can be carried out by comparing the values of the Gibbs energy of formation of A1N and MN.
The research carried out made it possible to develop a method for adequately constructing state diagrams of multicomponent nitrogen-containing systems and to establish the following patterns. At high concentrations of nitrogen and aluminum, the most informative method is the nitriding of powders of binary metal alloys at elevated nitrogen pressure. It was found that the optimal pressure is several tens of atmospheres.
In alloys based on transition metals and with low nitrogen content, the best results are obtained by methods of long-term homogenizing annealing and diffusion pairs. Distinctive feature The latter is the possibility of obtaining a large amount of data on the conditions of phase equilibrium when studying one sample. The commonly used technique for annealing powder compacts requires long-term isothermal exposure and at temperatures below 1473 - 1573 K, in many cases, does not allow achieving an equilibrium state of the alloy.
Experimental study of phase equilibria in alloys with low nitrogen content is in many cases difficult or even impossible due to the low accuracy of determining its concentration existing methods. For such sections of phase diagrams, it is effective to use methods of thermodynamic modeling and calculation of phase equilibria. They, based on data on phase equilibrium conditions found for more experimentally accessible sections of the phase diagram and available information on thermodynamic functions, make it possible to unambiguously establish the missing information. When solving a given problem, the corresponding system of equations, as a rule, turns out to be overdetermined, so the calculation not only makes it possible to establish the position of the equilibrium lines, but also to obtain comprehensive evidence of the adequacy of the solution. Thus, when carrying out thermodynamic calculations for all studied systems, the result did not depend on which experimentally found phase fields were used as initial data.
Another important area of using thermodynamic modeling and calculation is predicting experimental conditions and choosing the initial compositions of samples in such a way as to achieve the same final state of the alloy in different ways and prove its compliance with equilibrium.
In this work, using a complex of modern methods of physicochemical analysis, four isothermal sections of the state diagrams of ternary systems T1 - A1 - N. bm - A1 - N. W - A1 - N and N> - A1 - N at 1273 K are constructed. For this An approach based on the implementation of different paths to achieve the same final state of the alloy is consistently applied. The data found using various techniques are in good agreement both with each other and with the results of thermodynamic analysis, and therefore can be recommended for predicting phase equilibria in these systems and compositions based on them.
List of sources dissertation and abstract in physics, candidate of physical and mathematical sciences, Han Yu Xing, Moscow
1. Yoshimori Shigeru, Mizushima Kazuhiko, Kobayashi Akira, Takei Shu, Uchida Yasutaka, Kawamura Mitsuo. Synthesis and AES analysis of Nb(NbN)-AlN multilayers by off-axial DC magnetron sputtering. //Physica C. 1998. V.305(3&4), p.281-284.
2. Kwang Ho Kim, Seong Ho Lee. Structural Analyzes and Properties of Tii-XA1XN Films Deposited by PACVD using a TiCl4/AlCl3/N2/Ar/H2 Gas Mixture. //J. Cor. Cer. Soc. 1995. V.32. No.7, p.809-816.
3. Chen Kexin, Ge Changchun, Li Jiangtao. Phase formation and thermodynamic analysis of self-propagating high-temperature synthesis Al-Zr-N system composites. III. Mater. Res. 1998. V.13(9), p.2610-2613.
4. J.C. Schuster, J. Bauer, H. Nowotny. Applications to materials science of phase diagrams and crystal structures in the ternary systems transition metal-aluminum-nitrogen. //Revue de Chimie Minerale. 1985. T.22. p.546-554.
5. Murray J.L. Al-Ti (Aluminum-Titanium). //Binary Alloy Phase Diagrams, Second Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V.l, p.225-227.
6. Spencer P.J. Development of Thermodynamic Databases and Their Relevance for the Solution of Technical Problems. HZ. Metallkd. 1996. V.87, p.535-539.
7. Huang S.C., Siemers P.A. Characterization of the High-Temperature Phase Fields near Stoichiometric y-TiAl. //Metallurgical Transactions, Section A: Physical Metallurgy and Materials Science. 1989. V.20, p. 1899-1906.
8. Kaltenbach K., Gama S., Pinatti D.G., Schulze K.A. Contribution to the Al-Ti Phase Diagram. //Z. Metallkd. 1989. V.80, p.511-514.
9. Kornilov I.I., Pylaeva E.N., Volkova M.A., Kripyakevich P.I., Markiv V.Ya. Phase structure of alloys of the Ti-Al binary system containing from 0 to 30% AI. //Reports of the USSR Academy of Sciences. 1965. 161. No. 4, pp. 843-846.
10. Böhm N., Löhberg K. Über eine Überstrukturphase vom CsCl-Typ im System Titan-Molybdän-Aluminium. //Z. Metallkd. 1958. V.49, p. 173-178.
11. Sagel K., Schulz E., Zwicker U. Untersuchungen am System Titan-Aluminium. HZ. Metallkd. 1956. V.47, p.529-534.
12. McPherson DJ., Hansen M. Der Aufbau Binarer Legierungssysteme des Titans. HZ. Metallkd. 1954. V.45, p.76-81.
13. Bumps E. S., Kessler H. D., Hansen M. Titanium-Aluminium System, // Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. 1952. V.194. p.609-614.
14. Kornilov I.I., Pylaeva E.N., Volkova M.A. Diagram of the state of a titanium-aluminium binary system. //Izv. Academy of Sciences of the USSR. Dept. Chem. n. 1956. T.7, pp.771-777.
15. Kornilov I.I., Pylaeva E.N., Volkova M.A. Review of studies on the phase diagram of the Ti-Al binary system. //Titanium and its alloys. M. USSR Academy of Sciences. 1963. pp.74-85.
16. Murray J.L. Calculation of the Titanium-Aluminum Phase Diagram. //Metallurgical Transactions A. 1988. V.19A, p.243-247.
17. H. Okamoto. Ti Al. //J. Phase Equilibria. 1993. V.14, p.120.
18. Ogden H.R., Maykuth D.J., Finlay W.L., Jaffee R.I. Constitutions of Titanium-Aluminum Alloys. //Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. 1951. V. 191. p. 1150-1155.
19. Anderson C.D., Hofmeister W.H., Bayuzick R.J. Liquidus Temperatures in the Ti-Al System. //Metallugical Transactions A. 1993. V.24, p.61-66.
20. Kattner U.R., Lin J.C., Chang Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. //Metallurgical Transactions A. 1992. V.23, p.2081-2090.
21. Perepezko J.H. Phase Stability and Processing of Titanium Aluminides. //Proceedings of the International Symposium on Intermetallic Compounds, Structure and Mechanical Properties, (JIMIS-6). Sendai, Japan. 1991. p.239-243.
22. Perepezko J.H., Mishurda J.C. Phase Equilibria in the Titanium Aluminum System, //Titanium "92: Sci. and Technol.: Proc. Symp. 7th World Titanium Conf., San Diego, Calif., June 29 - July 2. 1992. V.l. Warrendale (Pa). 1992. p.563-570.
23. McCullough C., Valencia J.J., Levi C.G., Mehrabian R. Phase Equilibria and Solidification in Ti-Al Alloys. //Acta Metallurgies 1989. V.37, p. 1321-1336.
24. Chang J.Y., Moon I.G., Choi C.S. Microstructures of Heated Gamma(y)-Based Titanium-Aluminides. //J. Korean Inst. Met. & Mater. 1995. V.33. 11, p.1552-1561.
25. Collings E.W. Magnetic Studies of Phase Equilibria in Ti-Al (30 to 57 at.%) Alloys. //Metallurgical Transaction A. 1979. V.l OA. No. 4, p.463-473.
26. Jung I.S., Kim M.C., Lee J.H., Oh M.H., Wee D.M. Phase Equilibria of Ti-Al Alloy by Directional Solidification. //J. Cor. Inst. Met. & Mater. 1999. V.37. No. 4, p.448-453.
27. Jung I.S., Kim M.C., Lee J.H., Oh M.H., Wee D.M. High Temperature Phase Equilibria near Ti-50 at.% AI Composition in Ti-Al System Studied by Directional Solidification. //Intermetallics. 1999. V.7, p.1247-1253.
28. Okamoto H. Aluminum-Titanium. //J. Phase Equilibria. 2000. V. 21. No. 3, p. 311.
29. Zhang F., Chen S.L., Chang Y.A., Kattner U.R. A Themodynamic description of the Ti-Al system. //Intermetallics. 1997. V.5, p.471-482.
30. Kornilov I.I., Nartova T.T., Chernysheva S.P. About the phase diagram of Ti-Al in the titanium-rich part. //Izv. Academy of Sciences of the USSR. Metals. 1976. No. 6, p. 192-198.
31. Tsujimoto T., Adachi M. Reinvestigation of the Titanium Rich Region of the Titanium - Aluminum Equilibrium Diagram. //J. Institute of Metals. 1966. V.94. No. 10, p.358-363.
32. Van Loo F.J.J., Rieck G.D. Diffusion in the Titanium-Aluminum System II: Interdiffusion in the Composition Range between 25 and 100 at.% Ti. //Acta Metal. 1973. V.21, p.73-84.
33. Clark D., Jepson K.S., Lewis G.I. A Study of the Titanium -Aluminum System up to 40 at. % Aluminum. //J. Institute of Metals. 1962/63. V.91. No. 6, p. 197-203.
34. Sato T., Haung Y.C. The Equilibrium Diagram of the Ti-Al System. //Transactions of the Japan Institute of Metals. 1960. V.l, p.22-27.
35. Suzuki A., Takeyama M., Matsuo T. Transmission Electron Microscopy on the Phase Equilibria among ß, a and a2 Phases in Ti-Al Binary System. //Intermetallics. 2002. V.10, p.915-924.
36. Raman A., Schubert K. Uber den Aufbau Eunuger zu TiAb Verwandter Legierungsreihen. II. Untersuchungen in einigen Ti-Al-Si- und T4" 6 In-Systemen. HZ Metallkd. 1965. V.56, p.44-52.
37. Palm M., Zhang L.C., Stein F., Sauthoff G. Phase and Phase Equilibria in the Al-rich Part of the Al-Ti System above 900°C. //Intermetallics. 2002. V.10, p.523-540.
38. Schuster J.C., Ipser H. Phases and Phase Relations in the Partial System TiAh-TiAl. HZ. Metallkd. 1990. V.81, p.389-396.
39. Loiseau A., Vannffel C. TiAl2 a Reentrant Phase in the Ti AI System. //Phys. status solidi. 1988.V.l07. No. 2, p.655-671.
40. Hori S., Tai H., Matsumoto E. Solubility of titanium in aluminum in the solid state. //J. Japan Institute Light Metals. 1984. V.34. No. 7, p.377-381.
41. Abdel H.A., Allibert C.H., Durand F. Equilibrium between TiAh and Molten AI: Results from the Technique of Electromagnetic Phase Separation. //Z. Metallkd. 1984. V.75, p.455-458.
42. Minamino Y., Yamane T., Araki H., Takeuchi N., Kang Y., Miyamoto Y., Okamoto T. Solid Solubilities of Manganese and Titanium in Aluminum at 0.1 MPa and 2.1 Gpa. //Metallurgical Transactions A. 1991. V.22, p.783-786.
43. Liu Y.C., Yang G.C., Guo X.F., Huang J., Zhou Y.H. Coupled Growth Behavior in Rapidly Solidified Ti Al Peritectic Alloys. //J. Crystal Growth. 2001. V.222, p.645-654.
44. Mrowietz M., Weiss A. Solubility of Hydrogen in Titanium Alloys: I. The Solubility of Hydrogen in the System Tii-xGax, 0 45. Knapton A.G. The System Uranium-Titanium. //J. Institute of Metals. 1954/55. V.83, p.497-504. 46. Jamieson J.C. Crystal Structures of Titanium, Zirconium and Hafnium at High Pressures. //Science (Washington D.C.). 1963. V.140, p.72-73. 47. Sridharan S., Nowotny H. Studies in the Ternary System Ti-Ta-Al and in the Quaternary System Ti-Ta-Al-C. //Z. Metallkd. 1983. V.74, p.468-472. 48. Braun J., Ellner M. X-ray High-Temperature in-situ Investigation of the Aluminide TiAh (HfGa2 type). //J. Alloys and Compounds. 2000. V.309, p.l 18-122. 49. Braun J., Ellher M., Predel B. Zur Struktur der Hochtemperaturphase Ti-Al. //J. Alloys and Compounds. 1994. V.203, p.189-193. 50. Kumar K.S. X-Ray Peak Intensifies for the Binary Compound AljTi. //Powder Diffraction. 1990. V.5, p.165-167. 51. Bandyopadhyay J., Gupta K.P. Low Temperature Lattice Parameters of Al and Al Zn Alloys and Gruneisen Parameter of Al. //Cryogenics. 1978. V.l 8, p.54-55. 52. Kulikov I.S. Thermodynamics of carbides and nitrides. Chelyabinsk: Metallurgy, 1988.319p. 53. Peruzzi A., Abriata J.P. Al-Zr (Aluminum-Zirconium). //Binary Alloy Phase Diagrams, Second Edition Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V.l, p.241-243. 54. Murray J.L., McAlister A.J., Kahan D.J. The Al-Hf (Aluminum-Hafnium) System. //J. Phase Equilibria. 1998. No. 4, p.376-379. 55. Peruzzi A. Reinvestigation of the Zr-rich End of the Zr-Al Equilibrium Phase Diagram. //J. Nuclear Materials. 1992. V.186, p.89-99. 56. Sauders. N. Calculated Stable and Metastable Phase Equilibria in Al-Li-Zr Alloys. //Z. Metallkd. 1989. V.80, p.894-903. 57. Saunders N., Rivlin V.G. Thermodynamic Characterization of Al-Cr, Al-Zr, and Al-Cr-Zr Alloy Systems. //Materials Science and Technology. 1986. V.2, p.521-527. 58. Kaufman L., Nesor H. Calculation of the Ni-Al-W, Ni-Al-Hf and Ni-Cr-Hf Systems. //Canadian Metallurgical Quarterly. 1975. V.14, p.221-232. 59. Balducci G., Ciccioli A., Cigli G., Gozzi D., Anselmi-Tamburini U. Thermodynamic study of intermetallic phases in the Hf-Al system. //J. Alloys and Compounds. 1995. V.220, p. 117-121. 60. Matkovic P., Matkovic T., Vickovic I. Crystalline Structure of the Intermetallic Compound FeZr3. //Metallurgya. 1990. V.29, p.3-6. 61. Savitsky E.M., Tylkina M.A., Tsyganova I.A. Phase diagram of the zirconium - rhenium system. //Atomic Energy. 1959. V.7, pp. 724-727. 62. Ming L., Manghnani M.N., Katahara K.W. Investigation of a->x Transformation in the Zr-Hf System to 42 GPa, //J. Applied Physics. 1981. V.52, p.1332-1335. 63. Meng W.J., Faber J.jr., Okamoto P.R., Rehn L.E., Kestel B.J., Hitterman R.L. Neutron Diffraction and Transmission Electron Microscopy Study of Hydrogen-Induced Phase Transformations in Zr3Al. //J. Applied Physics. 1990. V.67, p.l 312-1319. 64. Clark N.J., Wu E. Hydrogen Absorption in the Zr-Al System. //J. Less-Common Metals. 1990. V. 163, p.227-243. 65. Nowotny H., Schob O., Benesovsky F. Die Kristallstruktur von Zr2Al und Hf2Al. //Monatshefte fur Chemie. 1961. V.92, p.1300-1303. 66. Nandedkar R.V., Delavignette P. On the Formation of a New Superstructure in the Zirconium-Aluminium System. //Physica Status Solidi A: Applied Research. 1982. V.73, p.K157-K160. 67. Kim S.J., Kematick R.J., Yi S.S., Franzen H.F. On the Stabilization of Zr5Al3 in the Mn5Si3-Type Structure by Interstitial Oxygen. //J. Less-Common Metals. 1988. V.137, p.55-59. 68. Kematick R.J., Franzen H.F. Thermodynamic Study of the Zirconium-Aluminum System. //J. Solid State Chemistry. 1984. V.54, p.226-234. 69. Hafez M., Slebarski A. Magnetic and Structural Investigations of Zri.xGdxAl2 Alloys. //J. Magnetism and Magnetic Materials. 1990. V.89, p. 124-128. 70. Desch P.B., Schwarz R.B., Nash P. Formation of Metastable Lb Phases in Al3Zr and Al-12.5% X-25% Zr(X=Li,Cr,Fe,Ni,Cu). //J. Less-Common Metals. 1991. V.168, p.69-80. 71. Ma Y., Romming C., Lebech V., Gjonnes J., Tafto J. Structure Refinement of Al3Zr using Single-Crystal X-ray Diffraction, Powder Neutron Diffraction and CBED. //Acta Crystallographica B. 1992. V.48, p. 11-16. 72. Schuster J.C., Nowotny H. Investigations of the Ternary Systems (Zr, Hf, Nb, Ta)-Al-C and Studies on Complex Carbides. //Z. Metallkd. 1980. V.71, p.341-346. 73. Maas J., Bastin G., Loo F.V., Metselaar R. The Texture in Diffusion-Grown Layers of . Trialuminides MeAl3 (Me=Ti, V, Ta, Nb, Zr, Hf) and VNi3. //Z Metallkd. 1983. V.74, p.294-299. 74. Wodniecki P., Wodniecka V., Kulinska A., Uhrmacher M., Lieb K.P. The Hafnium Aluminides HfAl3 and Н£гА13 Studied by Perturbed angular Corrlations with 181 Ta and mCd Probes. //J. Alloys and Compounds. 2000. V.312, p. 17-24. 75. Kuznetsov G.M., Barsukov A.D., Abas M.I. Study of the solubility of Mn, Cr, Ti and Zr in aluminum in the solid state. //Izv. universities Color Metallurgy. 1983. No. 1, pp. 96-100. 76. Rath V.V., Mohanty G.P., Mondolfo L.F. The Aluminum-Rich End of the Aluminum-Hafnium Diagram. //J. Institute of Metals. 1960/61. V.89, p.248-249. 77. Kattner U.R. AlNb. //Binary Alloy Phase Diagrams, second edition, Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V. 1, p. 179-181. 78. Suyama Ryuji, Kimura Masao, Hashimoto Keizo. Phase Stability and Fundamental Properties of Nb-Al Binary System. //Struct. Intermetallics. 1st Int. Symp. Struct. Intermetallics, Champion, Pa., Sept. 26-30, 1993, Warrendale (Pa). 1993. p.681-689. 79. Richards M.J. Contribution a l "etude du Systeme Niobiom-Aluminum. // Mémoires Scientifiques de la Revue de Metallurgie. 1964. V.61, p.265-270. 80. Herold A., Forsterling G., Kleinstuck K. Influence of the Real Structure on the Linear Thermal Expansion Coefficient of A15-type Intermetallic Compounds from Room Temperature to 10K. //Crystal Research and Technology. 1981. V. 16, p. 1137-1144. 81. Jorda J.L., Flukiger R., Muller J. A New Metallurgical Investigation of the Niobium-Aluminum System. //J. Less Common Metals. 1980. V.75, p.227-239. 82. Alfeu S.R., Carlos A.N. The Effect of Excess Aluminum on the Composition and Microstructure of Nb-Al Alloys Produced by Aluminothemic Reduction of Nb20s. //J. Materials Synthesis and Processing. 1999. V.7. No. 5, p.297-301. 83. Ahn I.S., Kim S.S., Park M.W., Lee K.M. Phase Characteristics of Mechanically Alloyed AI-10wt.%Nb Alloy. //J. Materials Science Letters. 2000. V.19, p.2015-2018. 84. Menon E.S.K., Subramanian P.R., Dimiduk D.M. Phase Transformations in Nb-Al-Ti Alloys. //Metallurgical Transaction A. 1996. V.27. No. 6, p. 1647-1659. 85. Kaufman L. Calculation of the Multicomponent Tantalum based Phase Diagrams. //CALPHAD. 1991. V. 15. No. 3, p.261-282. 86. Wriedt H.A. The Al-N (Aluminum-Nitrogen) System. //Bulletin of Alloy Phase Diagrams. 1986. V.7. No. 4, p.329-333. 87. Jones R.D., Rose K. Liquidus Calculations for III-IV Semiconductors. //CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry. 1984. V.8, p.343-354. 88. Hillert M., Josson S. An Assessment of the Al-Fe-N System. //Metallurgical Transaction A. 1992. V.23A, p.3141-3149. 89. Wriedt H.A., Murray J.L. N-Ti (Nitrogen-Titanium). //Binary Alloy Phase Diagrams, Second Edition, Ed. Massalski, ASM International, Materials Park, Ohio. 1990. V.3, p.2705-2708. 90. Zeng K., Schmid-Fetzer R. Critical Assessment and Thermodynamic Modeling of the Ti-N System. //Z. Metallkd. 1996.V.87. No. 7, p.540-554. 91. Etchessahar E., Bars J.P., Debuigne J. The Ti - N System: Equilibrium Between the Ô, e and a Phase and the Conditions of Formation of the Lobier and Marcon Metastable Phase. //J. Less-Common Metals. 1987. V.134, p. 123-139. 92. Vahlas C., Ladouce B.D., Chevalier P.Y., Bernard C., Vandenbukke L. A Thermodynamic Evaluation of the Ti N System. //Thermochemica Acta. 1991. V 180, p.23-37. 93. Etchessaher E., Sohn Y.U., Harmelin M., Debuigne J. The Ti N System: Kinetic, Calorimetric, Structure and Metallurgical Investigations of the ô-TiNo.si Phase. //J. Less-Common Metals. 1991. V. 167, p.261 -281. 94. Gusev A.I. Phase diagrams of ordered nonstoichiometric hafnium carbide and titanium nitride. //Reports of the Academy of Sciences. 1992. V.322. No. 5, pp. 918-923. 95. Gusev A.I., Rempel A.A. Phase diagrams of Ti C and Ti - N systems and atomic ordering of non-stoichiometric titanium carbide and nitride. //Reports of the Academy of Sciences. 1993. T.332. No. 6, pp. 717-721. 96. Lengauer W., Ettmayer P. Investigation of Phase Equilibria in the Ti N and Ti - Mo - N Systems. //Materials Science and Engineering A: Structure Materials: Properties, Microstructure and Processing. 1988. V.105/106. p.257-263. 97. Lengauer W. The Titanium Nitrogen System: A Study of Phase Reactions in the Subnitride Region by Means of Diffusion Couples. //Acta Metallurgica et Materialia. 1991. V.39, p.2985-2996. 98. Jonsson S. Assessment of the Ti N System. //Z. Metallkd. 1996.V.87. No. 9, p.691-702. 99. Ohtani H., Hillert M. A Thermodynamic Assessment of the Ti N System. //CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry. 1990. V.14, p.289-306. 100. Etchessahar E., Bars J.P., Debuigne J., Lamane A.P., Champin P. Titanium Nitrogen Phase Diagram and Diffusion Phenomena. //Titanium: Science and Technology Process 5 Int. Conf. Munich. Sept. 10-14 1984, V.3, Oberursel. 1985. p.1423-1430. 101. Wood F.W., Romans P.A., McCune R.A., Paasche O. Phases and Interdiffusion between Titanium and its Mononitride. //Rep. Infest. Bur. Mines. U.S. Dep. Inter. 1974. No. 7943. ii, p.40. 102. Em B.T., Latergaus I.S., Loryan V.E. Construction of the boundary of the region of existence of a solid solution of nitrogen in a-Ti using the neutron diffraction method. //Inorganic Mater. 1991. V.27. No. 3, pp. 517-520. 103. Kalmykov K.B., Rusina N.E., Dunaev S.F. Phase equilibria in the Al-Fe-Ni system at 1400K. //Vestn. Moscow Univ. Ser. 2. Chemistry. 1996. T.37. No. 5, pp. 469-473. 104. Toth L. Carbides and nitrides of transition materials. M.: Mir. 1974.294p. 105. Lengauer W. The Crystal Structure of ti-Ti3N2-x: An Additional New Phase in the Ti N System. //J. Less-Common Metals. 1996. V. 125, p. 127-134. 106. Christensen A.N., Alamo A., Landesman J.P. Structure of Vacancy-Ordered Titanium Heminitride 6"-Ti2N by Powder Neutron Diffraction. //Acta Crystallographica. Section C: Crystal Structure Communications. 1985. V.41, p.1009-1011. 107. Holmberg B. Structure Studies on the Titanium Nitrogen System. //Acta Chemica Scandinarica. 1962. V.16, p.1255-1261. 108. Lengauer W., Ettmayer P. The Crystal Structure of a New Phase in the Titanium-Nitrogen System. //J. Less-Common Metals. 1986. V.120, p.153-159. 109. Jiang C., Goto T., Hirai T. Non-stoichiometry of Titanium Nitride Plates Prepared by Chemical Vapor Deposition. //J. Alloys and Compounds. 1993. V.190, p. 197-200. 110. Eliot D.F., Glaser M., Ramakrishna V. Thermochemistry of steelmaking processes. M.: Metallurgy. 1969. 252 p. 111. Levinsky Yu.V. p-T State diagram of the zirconium-nitrogen system. //Physical chemistry. 1974. T.48, pp.486-488. 112. Domagala R.F., McPherson D.J., Hansen M. System Zirconium-Nitrogen. //Transaction of the American Institute of Mining, Metallurgical and Petroleum Engineering. 1956. V.206, p.98-105. 113. Massalski T.B. N-Zr. //Binary Alloy Phase Diagrams, Second Edition, Ed. T.B. Massalski, ASM International Materials Park, Ohio. 1990. V.3, p.2716-2717. 114. Ogawa T. Structural Stability and Thermodynamic Properties of Zr-N Alloys. //J. Alloys and Compounds. 1994. V.203, p.221-227. 115. Kosukhin V.B., Funke V.F., Minashkin V.L., Smirnov V.S., Efremov Yu.P. Preparation of coatings from zirconium nitride and carbonitride by CVD method. //Inorganic materials. News of the USSR Academies of Sciences. 1987. V.23, pp.52-56. 116. Lerch M., Fuglein E., Wrba J. Systhesis, Crystal Structure and High Temperature Behavior of Zr3N4. Z. Anorganische und Allgemeine Chemie. 1996. 622, p.367-372. 117. Massalski T.B. Hf-N. //Binary Alloy Phase Diagrams, Second Edition, Ed. T.B. Massalski, ASM Inter. Materials Park, Ohio. 1990*. V.2, p.2090-2092. 118. Christensen A.N. A Neutron Diffraction Investigation on Single Crystals of Titanium Oxide, Zirconium Carbide and Hafnium Nitride. //Acta Chemica Scandinavica. 1990. V.44, p.851-852. 119. Lengauer W., Rafaja D., Taubler R., Ettmayer P. Preparation of Binary Single-Phase Line Compounds via Diffusion Couples: The Subnitride Phase and C-Hf4N3.x. //Acta Metallurgica et Materialia. 1993. V.41, p.3505-3514. 120. Levinsky Yu.V. p-T State diagram of the niobium-nitrogen system. //Metals. 1974. V.1, pp. 52-55. 121. Huang W. Thermodynamic Properties of the Nb W - C - N System. //Z. Metallkd. 1997. V.88, p.63-68. 122. Lengauer W., Bohn M., Wollein V., Lisak K. Phase Reactions in the Nb N System Below 1400"C. //Acta Materialia. 2000. V.48, p.2633-2638. 123. Berger R., Lengauer W., Ettmayer P. The y-Nb4N3±x - 5-NbNi.x Phase Transition. //J. Alloys and Compounds. 1997. V.259, p.L9-L13. 124. Jogiet M., Lengauer W., Ettmayer P. III. Alloys and Compounds. 1998. V.46(2), p.233. 125. Huang W. Thermodynamic Assessment of the NbN System. //Metallurgical and Materials Transactions A. 1996. V.27A, p.3591-3600. 126. Balasubramanian K., Kirkaldy J.S. Experimental Investigation of the Thermodynamics of Fe-Nb-N austenite and Nonstoichiometric Niobium Nitride (1373-1673K). //Canadian Metallurgical Quarterly. 1989. V.28, p.301-315. 127. Christensen A.N. Preparation and Crystal Structure of ß-Nb2N and y-NbN. //Acta Chemica Scandinavica, A: Physical and Inorganic Chemistry. 1976. V.30, p.219-224. 128. Christensen A.N., Hazell R.G., Lehmann M.S. An X-ray and Neutron Diffraction Investigation of the Crystal Structure of y-NbN, //Acta Chemica Scandinavica, A: Physical and Inorganic Chemistry. 1981. V.35, p.l 11-115. 129. Lengauer W., Ettmayer P. Preparation and Properties of Compact Cubic 5-NbNi-x. //Monatshefte fur Chemie. 1986. V.l 17, p.275-286. 130. Yen C.M., Toth L.E., Shy Y.M., Anderson D.E., Rosner L.G. Superconducting Hc-Jc and Tc Measurements in the Nb-Ti-N, Nb-Hf-N and Nb-V-N Ternary Systems. //J. Applied Physics. 1967. V.38, p.2268-2271. 131. Terao N. New Phases of Niobium Nitride. //J. the Less-Common Metals. 1971. V.23, p.159-169. 132. Dobrynin A.B. New aluminum nitride ceramic materials. //Inorganic materials. 1992. V.28. No. 7, pp. 1349-1359. 133. Kulikov V.I., Mushkarenko Yu.N., Parkhomenko S.I., Prokhorov L.N. A new class of ceramic materials based on thermally conductive aluminum nitride. //Electronic equipment. Ser. Microwave Technology. 1993. T.2(456), pp.45-47. 134. Samsonov G.V. Nitrides. Kyiv: Naukova Dumka. 1969. 377 p. 135. Kral S., Lengauer W., Rafaja D., Ettmayer P. Critical Review on the Elastic Properties of Transition Metal Carbides, Nitrides and Carbonitrides. IIJ. Alloys and Compounds. 1998. V.265, p.215-233. 136. Samsonov G.V., Pilipenko A.T., Nazarchuk T.N. Analysis of refractory compounds. M: Metallurgizdat. 1962. 256 p. 137. Samonov G.V., Strashinskaya J1.B., Schiller E.A. Contact interaction of metal-like carbides, nitrides and borides with refractory metals at high temperatures. //Metallurgy and fuel. 1962. V.5, pp. 167-172. 138. Dai Ying, Nan Ce-wen. Synthesis of aluminum nitride whiskers via a vapor-liquid-solid process, //material Res. Soc. Symp. Proc. 1999. V.547, p.407-411. 139. Chen K.X., Li J.T., Xia Y.L., Ge C.C. Self-propagating high-temperature synthesis (SHS) and microstructure of aluminum nitride. //Int. J. Self-Propagating High-Temp. Synthesis. 1997. V.6(4), p.411-417. 140. Hwang C.C., Weng C.Y., Lee W.C., Chung S.L. Synthesis of A1N powder by a combustion synthesis method. //Int. J. Self-Propagating High-Temp. Synthesis. 1997. V.6(4), p.419-429. 141. Chung S.L., Yu W.L., Lin C.N. A self-propagating high-temperature synthesis method for synthesis of A1N powder. //J. Material Research. 1999. V.14(5), p. 1928-1933. 142. Ha H., Kim K.R., Lee H.C. A Study on the Synthesis of Titanium Nitride by SHS (Self-propagating High-temperature Synthesis) Method. //J. Cor. Ceramic. Soc. 1993. V.30. No. 12, p. 1096-1102. 143. Chen K., Ge C., Li J. Phase formation and thermodynamic analysis of self-propagating high-temperature synthesis Al-Zr-N system composites. //J. Material Research. 1998. V.13(9), p.2610-2613. 144. Chen K.X., Ge C.C., Li J.T. The effect of nitrogen pressure on in situ combustion synthesis of AIN-ZrN composites. //Metallurgical. Materials. Trans. A, 1999. V.30A(3A). p.825-828. 145. Garcia I., Olias J.S., Vazquez A.J. A new method for materials synthesis: solar energy concentrated by Fresnel lens. //J. Physics. 1999.IV. V.9. p.Pr3/435-Pr3/440. 146. Olias J.S., Garcia I., Vazquez A.J. Synthesis of TiN with solar energy condensed by a Fresnel lens. //J. Material Letters. 1999. V.38, p.379-385. 147. Boulmer-Leborgne C., Thomann A.L., Andreazza-Vignolle P., Hermann J., Craciun V., Echegut P., Crariun D. Excimer laser synthesis of A1N coating. //Appl. surface science. 1998. V. 125, p. 137-148. 148. Sicard E., Boulmer-Leborgne C., Sauvage T. Excimer laser induced surface nitriding of aluminum alloy. //Appl. Surface Science. 1998. V.127-129, p.726-730. 149. Boulmer-Leborgne C., Thomann A.L., Hermann J. Direct synthesis of metal nitride by laser. //NATO ASI Ser. 1996. Ser.E. V.307, p.629-636. 150. Thomann A.L., Sicard E., Boulmer-Leborgne C., Vivien C., Hermann J., Andreazza-Vignolle C., Andreazza P., Meneau C. Surface nitriding of titanium and aluminum by laser-induced plasma. //Surface Coating Technology. 1997.V.97. No.(1-3), p.448 452. 151. Dai X., Li Q., Ding M., Tian J. Thermodynamic aspect in synthesis of A1N powders by carbothermal reduction and nitridation process. //J. Material. Science. Technology. 1999. V.15(l), p.13-16. 152. Wang J., Wang W.L., Ding P.D., Yang Y.X., Fang L., Esteve J., Polo M.C., Sanchez G. Synthesis of cubic aluminum nitride by carbothermic nitridation reaction. //Diamond Relat. Mater. 1999. V.8(7), p. 1342-1344. 153. Pathak Lokesh Chandra, Ray Ajoy Kumar, Das Samar, Sivaramakrishnan C. S., Ramachandrarao P. Carbothermal synthesis of nanocrystalline aluminum nitride powders. //J. American Ceramic Society. 1999. V.82(l), p.257-260. 154. Clement F., Bastians P., Grange P. Novel low-temperature synthesis of titanium nitride: proposal for cyanonitridation mechanism. //Solid State Ionics. 1997. V.101-103. p.171-174. 155. Jung W.S., Ahn S.K. Synthesis of aluminum nitride by the reaction of aluminum sulfide with ammonia. //Materials Letters. 2000. V.43, p.53-56. 156. Hezler J., Leiberich R., Mick H.J., Roth P. Shock tube study of the formation of TiN molecules and particles. //Nanostruct. Materials. 1999. V.l 0(7), p. 1161-1171. 157. Uheda K., Takahashi M., Takizawa H., Endo T., Shimada M. Synthesis of aluminum nitride using urea-precursors. //Key Eng. Materials. 1999. V.l59-160, p.53-58. 158. Shimada S., Yoshimatsu M., Nagai H., Suzuku M., Komaki H. Preparation and properties of TiN and A1N films from alkoxide solution by thermal plasma CVD method. //Thin Solid Films. 2000. V.370, p.137-145. 159. Shimada S., Yoshimatsu M. Preparation of (Tii.xAlx)N films from mixed alkoxide solutions by plasma CVD. //Thin Solid Films. 2000. V.370, p.146-150. 160. Kim W.S., Sun H.N., Kim K.Y., Kim B.H. A study on the TiN Thin Film by Sol-Gel Method. //J. Cor. Ceramic. Soc. 1992. V.29. No. 4, p.328-334. 161. Sonoyama Noriyuki, Yasaki Yoichi, Sakata Tadayoshi. Formation of aluminum nitride using lithium nitride as a source of N3" in the molten aluminum chloride. //Chemical Letters. 1999. V.3, p.203-204. 162. Nakajima Kenichiro, Shimada Shiro. Electrochemical synthesis of TiN precursors and their conversion to fine particles. //J. Material Chem. 1998. V.8(4), p.955-959. 163. Pietzke M.A., Schuster J.C. Phase Equilibria of the Quaternary System Ti A1 - Sn - N at 900°C. //J. Alloys and Compounds. 1997. V.247, p. 198-201. 164. Schuster J.C., Bauer J. The Ternary System Titanium Aluminum - Nitrogen. //J. Solid State Chemistry. 1984. V.53, p.260-265. 165. Procopio A.T., El-Raghy T., Barsoum M.W. Synthesis of Ti4AlN3 and Phase Equilibria in the Ti - A1 N System. //Metallurgical and Materials Transactions A. 2000. V.31A, p.373-378. 166. Zeng K., Schmid-Fetzer R. Thermodynamic Modeling and Applications of the Ti A1 - N Phase Diagram. //Thermodynamics of alloy formation, 1997TMS annual meeting in Orlando, Florida, February 9-13. 1997. p.275-294. 167. Chen G., Sundman B. Thermodynamic Assessment of the Ti A1 - N System. //J. Phase Equilibria. 1998.V.19. No. 2, p.146-160. 168. Anderbouhr S., Gilles S., Blanquet E., Bernard C., Madar R. Thermodynamic Modeling of the Ti A1 - N System and Application to the Simulation of CVD Processes of the (Ti, A1)N Metastable Phase. //Chem.Vap.Deposition. 1999. V.5. No. 3, p.109-113. 169. Pietzka M.A., Schuster J.C. Phase Equilibria in the Quaternary System Ti A1 - C - N. //J. American Ceramic Society. 1996. V.79(9), p.2321-2330. 170. Lee H.D., Petuskey W.T. New Ternary Nitride in the Ti Al - N System. //J. American Ceramic Society. 1997. V.80. No. 3, p.604-608. 171. Ivanovskii A.L., Medvedeva N.I. Electronic Structure of Hexagonal Ti3AlC2 and Ti3AlN2. //Mendeleev Communications Electronic Version. 1999. V.l, p.36-38. 172. Barsoum M.W., Schuster J.C. Comment on "New Ternary Nitride in the Ti Al - N System". //J. American Ceramic Society. 1998.V.81. No. 3, p.785-789. 173. Barsoum M.W., Rawn C.J., El-Raghy T., Procopio A.T., Porter W.D., Wang H., Hubbard C.R. Thermal Properties of Ti4AlN3. //J. Applied Physics. 2000. V.87, p.8407-8414. 174. Procopio A.T., Barsoum M.W., El-Raghy T. Characterization of Ti4AlN3. //Metallurgical and Materials Transactions A. 2000. V.31A, p.333-337. 175. Myhra S., Crossley J.A.A., Barsoum M.W. Crystal-Chemistry of the Ti3AlN3 Layered Carbide/Nitride Phase-Characterization by XPS. III. Physics and Chemistry of Solids. 2001. V.62, p. 811-817. 176. El-Sayed M.H., Masaaki N., Schuster J.C. Interfacial Structure and Reaction Mechanism of AIN/Ti Joints. III. Materials Science. 1997. V.32, p.2715-2721. 177. Paransky Y., Berner A., Gotman I. Microstructure of Reaction Zone at the Ti A1N Interface. //Materials Letters. 1999. V.40, p. 180-186.9 178. Paransky Y.M., Berner A.I., Gotman I.Y., Gutmanas E.Y. Phase Recognition in A1N-Ti System by Energy Dispersive Spectroscopy and Electron Backscatter Diffraction. //Mikrochimica Acta. 2000. V.134, p.l71-177. 179. Gusev A.I. Phase equilibria in ternary systems M-X-X" and M-A1-X (M-transition metal, X, X" - B, C, N, Si) and crystal chemistry of ternary compounds. //Chemistry successes. 1996. V.65(5), pp.407-451. 180. Schuster J.C., Bauer J., Debuigne J. Investigation of Phase Equilibria Related to Fusion Reactor Materials: 1. The Ternary System Zr A1 - N. III. Nuclear Materials. 1983. V.116, p.131-135. 181. Schuster J.C. The Crystal Structure of Zr3AlN. //Z. Kristallography. 1986. V.175, p.211-215. 182. Schuster J.C., Bauer J. Investigation of Phase Equilibria Related to Fusion Reactor Materials: II. The Ternary System Hf-Al-N. III. Nuclear Materials. 1984. V.120, p.133-136. 183. Schuster J.C., Nowotny H. Phase Equilibria in the Ternary Systems Nb-Al-N and Ta-Al-N. //Z. Metallkd. 1985. V.76, p.728-729. 184. Jeitschko W., Nowotny H., Benesovsky F. Strukturchemische Unter Suchungen an Komplex -Carbiden und -Nitriden. //Monatsh Chem. 1964. V.95, p.l 56. 185. Reed S. Electron probe microanalysis. M.: Mir. 1979. 260 p. 186. Sokolovskaya E.M., Guzey JI.C. Metal chemistry. M.:Mosk. Univ. 1986. 264 p. 187. Abramycheva H.JI. Interaction of alloys based on iron, nickel and elements of groups IV–V with nitrogen at elevated partial pressure. Abstract of the candidate's dissertation, Moscow State University, 1999. 20 p. 188. Lupis K. Chemical thermodynamics of materials. M.: Metallurgy. 1989. 503 p. 189. Dinsdale A.T. SGTE Data for Pure Elements. //Calphad. 1991. V. 15. No. 4, p.317-425. 190. Kaufmann L., Nesor H. Coupled Phase Diagrams and Thermochemical Data for Transition Metal Binary Systems V. // Calphad. 1978. V.2. No. 4, p.325-348. 191. Voronin G.F. Partial thermodynamic functions of heterogeneous mixtures and their application in the thermodynamics of alloys. //In the book: Modern problems of physical chemistry. M.: Moscow. Univ. 1976. vol.9. p.29-48. 192. Kaufman L., Bershtein X. Calculation of state diagrams using a computer: Transl. from English M.: Mir. 1972. 326 p. 193. Belov G.V., Zaitsev A.I. Using the Monte Carlo method to determine the phase composition of heterogeneous systems. // Abstracts of the XIV International Conference on Chemical Thermodynamics. St. Petersburg: Scientific Research Institute of St. Petersburg State University. T.2002. p.317-318. 194. Khan Yu.S., Kalmykov K.B., Dunaev S.F., Zaitsev A.I. Phase equilibria in the Ti-Al-N system at 1273 K. // Reports of the Academy of Sciences. 2004. t.396. No. 6, pp. 788-792. 195. Han Y.S., Kalmykov K.V., Dunaev S.F., Zaitsev A.I. Solid-State Phase Equilibria in the Titanium-Aluminum-Nitrogen System. //J. Phase Equilibria and Diffusion. 2004. V.25. No. 5, p.427-436. 196. State diagrams of binary metal systems. Directory: In 3 volumes: T.Z. Book 1 /Under. General Ed. N.P. Lyakisheva. M.: Mechanical engineering. 1999. 880 p. 197. Wang T., Jin Z., Zhao J.C. Thermodynamic Assessment of the Al-Zr Binary System. //J. Phase Equilibria. 2001. V.22. No. 5, p.544-551. 198. Turkdogan E.T. Physical chemistry of high-temperature processes. M.: Metallurgy. 1985. 344 p. 199. Han Y.S., Kalmykov K.V., Abramycheva N.L., Dunaev S.F. Structure of the Al-Zr-N system at 1273K and 5Mpa. //VIII International conference of crystalchemistry of intermetallic compounds. Lviv. Ukraine. September 25-28.2002. p.65. 200. Khan Yu.S., Kalmykov K.B., Zaitsev A.I., Dunaev S.F. Phase equilibria in the Zr-Al-N system at 1273 K. //Metals. 2004. T.5, pp.54-63. 201. Han Yu Sin, Kalmykov K.B., Dunaev S.F. Interaction of aluminum nitride with elements of group IVB. //International Conference of Undergraduate and Postgraduate Students on Basic Sciences "Lomonosov-2003". April 15-18, 2003 section Chemistry. T.2, p.244. II. LITERATURE REVIEW. § 1. DUAL SYSTEMS OF ELEMENTS OF GROUPS IV - V WITH ALUMINUM. 1.1. Vehicle state diagram - A1. 1.2. The structure of the binary systems Bx - A1 and NG - A1. 1.3. Structure of the phase diagram of the binary system Lb - A1. § 2. STRUCTURE OF BINARY SYSTEMS M - N (M = A1, TC, Bx, Shch B). 2.1. State diagram A1 - N. 2.2. Vehicle condition diagram - N. 2.3. State diagrams of binary systems Bx - N and NG - N. 2.4. Phase diagram of Lb - N. 2.5. Physicochemical properties and methods of synthesis of nitrides. § 3. STRUCTURE OF TRIPLE STATE DIAGRAMS M - A1 - N M = TC, bx, H £ bb). 3.1. Vehicle state diagram - A1 - N. 3.2. State diagrams of Bx - A1 - N and NG- A1 - N. 3.3. State diagram N1) - A1 - N. III. EXPERIMENTAL PART § 1. METHODS OF SAMPLE PREPARATION. §2. METHODS FOR STUDYING SAMPLES. 2.1. Electron probe microanalysis (EPMA). 2.2. Scanning electron microscopy (SEM). 2.3. Optical microscopy. 2.4. X-ray phase analysis. § 3 DEVELOPMENT OF A METHOD FOR STUDYING PHASE DIAGRAMS WITH NITROGEN INVOLVED. IV. RESULTS AND DISCUSSION. § 1. PHASE EQUILIBRIA IN THE SYSTEM T1 - A1 - N. § 2. CONDITIONS FOR PHASE EQUILIBRIUM IN THE SYSTEM Bx - A1 - N. § 3. STRUCTURE OF THE STATE DIAGRAM OF THE SYSTEM W - A1 - N. dd § 4. PHASE EQUILIBRIA IN THE SYSTEM A - A1 - N. Ceramic materials based on double aluminum nitrides and group IV elements are widely used in various fields of industry and technology. In microelectronics, it is generally accepted to use substrates made of aluminum nitride, which has a unique combination of high properties: heat resistance, electrical resistance and thermal conductivity. Due to its resistance to metal melts, titanium nitride is promising for metallurgy. Zirconium nitride is an important component of nitride nuclear fuel in fast breeder reactors. Currently, significant interest is being paid to the development of various composite materials based on aluminum nitride in combination with nitrides of transition metals of groups IV - V. In particular, an important role in the development of microelectronics is assigned to multilayer material consisting of A1N and NbN layers. No less promising for creating wear-resistant and protective coatings, diffusion barriers in microelectronics, high-temperature ceramic, metal-ceramic, and composite materials are Ti - Al - N and Zr - Al - N alloys. Determination of the phase composition of such materials showed the presence of only double nitride phases. However, recent, thorough studies of M - Al - N alloys (hereinafter M = Ti, Zr, Hf, Nb) have revealed the existence of complex nitrides: Ti3AlN, TÎ2A1N, Ti3Al2N2; Zr3AlN, ZrsAbNj.x; Hf3AlN, Hf5Al3N; Nb3Al2N. Their properties have been practically unstudied, although there is good reason to believe that they may be unique. This is evidenced by the fact that composite materials based on a combination of double nitrides A1 and M have the maximum level of physical characteristics precisely in the areas of triple phase compositions. For example, the abrasive properties of Ti - Al - N ternary compounds are twice as high as those of corundum and even than those of tungsten carbide. An equally important role is played by compounds of A1 and elements of groups IV - V with nitrogen in the design and production of a wide range of grades of steels and alloys, especially with a high nitrogen content. Naturally, the physical, physicochemical and mechanical properties of the listed materials are directly related to the type and quantities of nitrogen-containing phases formed. Accurate data on the composition and conditions of existence of complex compounds are also of fundamental theoretical importance for understanding the nature of the chemical bond and other key characteristics that determine the degree of their stability. To predict the synthesis conditions and stability of nitrides, reliable information about phase equilibria is required. Constructing multicomponent phase diagrams with the participation of nitrogen is a very difficult task due to the low thermodynamic incentives for the formation of mixed compounds from double phases adjacent in the phase diagram, the low diffusion rates of components in them, as well as the complexity and low accuracy of determining the true nitrogen content. Therefore, the currently available information is fragmentary and extremely contradictory both regarding the composition of ternary nitrides and the position of the phase equilibrium lines. It was mainly obtained by one group of researchers by annealing powder compacts, in which achieving an equilibrium state of the alloy is difficult. GOAL OF THE WORK: Development of a new approach to the study of phase diagrams of multicomponent nitride systems, based on the use of a complex of modern experimental techniques of physicochemical analysis, methods of thermodynamic analysis and calculation, which makes it possible to determine with high accuracy the conditions for the coexistence of phases and obtain comprehensive evidence of their compliance with equilibrium. Study of phase equilibria in the solid-phase region of ternary systems aluminum - nitrogen - metal of IV - V groups at a temperature of 1273 K. SCIENTIFIC NOVELTY: Methods of thermodynamic analysis and calculations have been used to show the inconsistency of the available experimental data on the conditions of phase equilibrium in T1-Al-Ligg-Al-K systems; A methodology has been developed for studying the phase diagrams of nitride systems, which is based on a set of modern methods of physical and chemical analysis and the implementation of different ways to achieve the same final state of the alloy, which allows obtaining comprehensive evidence of compliance with its equilibrium; Thermodynamic modeling, analysis and calculation of phase equilibria in the systems Bx - A1 - N and NG - A1 - N were carried out. The thermodynamic functions of ternary compounds formed in these systems were found for the first time; The solid-phase regions of the state diagrams of the P - A1 - N systems are constructed. A1-S and NG-A1-S at 1273 K; The nature of phase equilibria in the Lib - Al - N system at a temperature of 1273 K has been established. SCIENTIFIC AND PRACTICAL SIGNIFICANCE OF THE WORK: The information obtained about the equilibrium conditions and thermodynamic functions of phases in systems M - A1 - N (M = T1, bx, H £ bb) is a fundamental scientific basis for the development of coatings, ceramic and metal-ceramic, composite materials, important for microelectronics, energy, and mechanical engineering . They make it possible to determine technological parameters for the production and processing of such materials, and are also of fundamental importance for predicting the phase composition and properties of a wide range of steels and alloys with a high nitrogen content. RELIABILITY AND VALIDITY: Data obtained by various methods of physicochemical analysis on samples of alloys synthesized by various methods (nitriding of binary alloys, long-term homogenizing annealing, diffusion pairs), using modern experimental approaches and equipment, such as electron probe microanalysis, scanning electron microscopy, X-ray phase analysis, in all cases were in excellent agreement both with each other and with the results of thermodynamic calculations. THE FOLLOWING PROVISIONS ARE MADE FOR DEFENSE: 1. A technique for constructing phase diagrams of multicomponent nitride systems, based on a combination of a set of modern methods of physical and chemical analysis with various ways to achieve the same equilibria, thermodynamic modeling and calculation of phase equilibria. 2. Structure of the solid-phase region of the isothermal section of the phase diagram “L - A1 - N at a temperature of 1273 K. 3. Results of thermodynamic analysis and calculation of phase equilibria in the Tl - A1 - N system at 1273 and 1573 K. 4. Structure of the solid-phase regions of the state diagrams of the systems Zg - A1 - N. NG- A1 - N. N1) - A1 - N at 1273 K. II. LITERATURE REVIEW VI. conclusions. 1. A methodology has been developed for studying the state diagrams of multicomponent nitride systems, based on a combination of methods of nitriding of binary alloys, long-term homogenizing annealing of three-component compositions, diffusion pairs, thermodynamic calculations and modeling of phase equilibria. It allows you to implement different ways to achieve the same final state of the alloy and obtain comprehensive evidence of compliance with its equilibrium. It has been established that when studying areas of state diagrams with high nitrogen concentrations, the most reliable and informative method is the nitriding method of binary alloys. At low nitrogen concentrations, the best results are obtained by the diffusion pair method. 2. Using modern approaches of thermodynamic calculation and modeling of phase equilibrium conditions, an analysis of existing data on the state diagrams of M-A1-I systems was carried out. Their inconsistency has been revealed and ways of optimal experimental research have been determined. 3. Using a complex of modern methods of physicochemical analysis, the patterns of interaction of elements in 85 samples of binary and ternary alloys of the M-A1-N systems were studied. 4. A solid-phase state diagram of the T1-A1-K system at 1273 K has been constructed. It has been established that aluminum nitride is in equilibrium with the phases IA13, "PgASH and TO^.*. The ternary compound TS3AIA forms three-phase regions with the phases TSgASH, T1A1, T13A1, a(P) and The parameters of the crystal lattices of the ternary phases T12ASh (a=2.986(9)A, c=13.622(5)A), T13ASh (a=4.1127(17)A), and the Gibbs energy of their formation from modifications of elements stable at this temperature: -360.0 kJ/mol and -323.3 kJ/mol, respectively. 5. Phase equilibria in crystalline alloys at 1273 K were studied. The position of all regions of three-phase equilibria was reliably established. Aluminum nitride is in equilibrium with the 2gAl3, ZmA\2 and ZgN phases. The triple phase ggzANYA forms fields of three-phase equilibria with phases ZrsAbNi.x and a(Zr)-based solid solution. The lattice parameters of the complex nitride Z^AIN are d=3.366(6)А, ¿»=11.472(10)В, c=8.966(9)В, Gibbs energy of formation А/3 = -380.0 kJ/mol. 6. It has been established that in solid compositions of the Hf-Al-N system at 1273 K, almost all double phases of the Hf-Al system are in equilibrium with hafnium nitride HfN. The ternary compound Hf^AlN forms regions of three-phase equilibrium with the HfsAh, HfN phases and the a(Hf)-based solid solution. Double phases Hf2Al, ^N2 occur only in limited compositional regions of the ternary system. Aluminum nitride is in equilibrium with HgAl3 and HfN. 7. For the first time, an isothermal T=1273 K section of the solid-phase part of the state diagram of the Nb-Al-N system was constructed. The ternary compound Nl^AhN is in equilibrium with the phases AIN, NbAb, NbAb and Nb2N. The Nb3Al-based phase and the niobium-based solid solution form a three-phase field with Nb2N. Niobium nitride NbN is in equilibrium with aluminum nitride and Nb2N. V. CONCLUSION. A general pattern in the structure of the phase diagrams of the studied M - Al - N systems is a decrease in the number and stability of complex nitride phases as the difference between the thermodynamic stability of the double phases MN and A1N increases, which is characterized by the Gibbs energy of formation Zl/7(A1N) = -180.0 kJ/mol, Zl/7(TiN)=-217.8 kJ/mol, 4G(ZrN)=-246.4 kJ/mol, ZlyG(HfN)-251.0 kJ/mol, zl/7(NbN) =-110.7 kJ/mol. Thus, in the systems Ti - Al - N and Zr - Al - N at 1273 K there are two complex nitrides TijAIN, Ti2AlN and Z^AIN, ZrsAbNi-x, respectively. Moreover, at high temperatures in Ti - Al - N alloys, the TÎ4A1N3.X phase is stable, and the ZrsAbNi-* compound cannot be considered ternary, since it is isostructural with the ZrsAb intermetallic compound. In the phase diagrams of Hf - Al - N and Nb - Al - N, there is only one complex compound Hf3AlN and Nb3Al2N, respectively. In the Ti - Al - N and Nb - Al - N systems, aluminum nitride is in equilibrium with the corresponding complex nitride, titanium or niobium nitrides and titanium or niobium aluminides with the maximum concentration of aluminum. In systems with zirconium and hafnium, the AIN - M3AIN equilibrium disappears. This is caused by an increase in the thermodynamic stability of the double nitride phases ZrN and HfN. Thus, predicting the possibility of obtaining three-component nitride phases, including in steels and alloys, can be carried out by comparing the values of the Gibbs energy of formation of A1N and MN. The research carried out made it possible to develop a method for adequately constructing state diagrams of multicomponent nitrogen-containing systems and to establish the following patterns. At high concentrations of nitrogen and aluminum, the most informative method is the nitriding of powders of binary metal alloys at elevated nitrogen pressure. It was found that the optimal pressure is several tens of atmospheres. In alloys based on transition metals and with low nitrogen content, the best results are obtained by methods of long-term homogenizing annealing and diffusion pairs. A distinctive feature of the latter is the possibility of obtaining a large amount of data on the conditions of phase equilibrium when studying one sample. The commonly used technique for annealing powder compacts requires long-term isothermal exposure and at temperatures below 1473 - 1573 K, in many cases, does not allow achieving an equilibrium state of the alloy. Experimental study of phase equilibria in alloys with low nitrogen content is in many cases difficult or even impossible due to the low accuracy of determining its concentration by existing methods. For such sections of phase diagrams, it is effective to use methods of thermodynamic modeling and calculation of phase equilibria. They, based on data on phase equilibrium conditions found for more experimentally accessible sections of the phase diagram and available information on thermodynamic functions, make it possible to unambiguously establish the missing information. When solving a given problem, the corresponding system of equations, as a rule, turns out to be overdetermined, so the calculation not only makes it possible to establish the position of the equilibrium lines, but also to obtain comprehensive evidence of the adequacy of the solution. Thus, when carrying out thermodynamic calculations for all studied systems, the result did not depend on which experimentally found phase fields were used as initial data. Another important area of using thermodynamic modeling and calculation is predicting experimental conditions and choosing the initial compositions of samples in such a way as to achieve the same final state of the alloy in different ways and prove its compliance with equilibrium. In this work, using a complex of modern methods of physicochemical analysis, four isothermal sections of the state diagrams of ternary systems T1 - A1 - N. bm - A1 - N. W - A1 - N and N> - A1 - N at 1273 K are constructed. For this An approach based on the implementation of different paths to achieve the same final state of the alloy is consistently applied. The data found using various techniques are in good agreement both with each other and with the results of thermodynamic analysis, and therefore can be recommended for predicting phase equilibria in these systems and compositions based on them. 1. Yoshimori Shigeru, Mizushima Kazuhiko, Kobayashi Akira, Takei Shu, Uchida Yasutaka, Kawamura Mitsuo. Synthesis and AES analysis of Nb(NbN)-AlN multilayers by off-axial DC magnetron sputtering. //Physica C. 1998. V.305(3&4), p.281-284. 2. Kwang Ho Kim, Seong Ho Lee. Structural Analyzes and Properties of Tii-XA1XN Films Deposited by PACVD using a TiCl4/AlCl3/N2/Ar/H2 Gas Mixture. //J. Cor. Cer. Soc. 1995. V.32. No.7, p.809-816. 3. Chen Kexin, Ge Changchun, Li Jiangtao. Phase formation and thermodynamic analysis of self-propagating high-temperature synthesis Al-Zr-N system composites. III. Mater. Res. 1998. V.13(9), p.2610-2613. 4. J.C. Schuster, J. Bauer, H. Nowotny. Applications to materials science of phase diagrams and crystal structures in the ternary systems transition metal-aluminum-nitrogen. //Revue de Chimie Minerale. 1985. T.22. p.546-554. 5. Murray J.L. Al-Ti (Aluminum-Titanium). //Binary Alloy Phase Diagrams, Second Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V.l, p.225-227. 6. Spencer P.J. Development of Thermodynamic Databases and Their Relevance for the Solution of Technical Problems. HZ. Metallkd. 1996. V.87, p.535-539. 7. Huang S.C., Siemers P.A. Characterization of the High-Temperature Phase Fields near Stoichiometric y-TiAl. //Metallurgical Transactions, Section A: Physical Metallurgy and Materials Science. 1989. V.20, p. 1899-1906. 8. Kaltenbach K., Gama S., Pinatti D.G., Schulze K.A. Contribution to the Al-Ti Phase Diagram. //Z. Metallkd. 1989. V.80, p.511-514. 9. Kornilov I.I., Pylaeva E.N., Volkova M.A., Kripyakevich P.I., Markiv V.Ya. Phase structure of alloys of the Ti-Al binary system containing from 0 to 30% AI. //Reports of the USSR Academy of Sciences. 1965. 161. No. 4, pp. 843-846. 10. Böhm N., Löhberg K. Über eine Überstrukturphase vom CsCl-Typ im System Titan-Molybdän-Aluminium. //Z. Metallkd. 1958. V.49, p. 173-178. 11. Sagel K., Schulz E., Zwicker U. Untersuchungen am System Titan-Aluminium. HZ. Metallkd. 1956. V.47, p.529-534. 12. McPherson DJ., Hansen M. Der Aufbau Binarer Legierungssysteme des Titans. HZ. Metallkd. 1954. V.45, p.76-81. 13. Bumps E. S., Kessler H. D., Hansen M. Titanium-Aluminium System, // Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. 1952. V.194. p.609-614. 14. Kornilov I.I., Pylaeva E.N., Volkova M.A. Diagram of the state of a titanium-aluminium binary system. //Izv. Academy of Sciences of the USSR. Dept. Chem. n. 1956. T.7, pp.771-777. 15. Kornilov I.I., Pylaeva E.N., Volkova M.A. Review of studies on the phase diagram of the Ti-Al binary system. //Titanium and its alloys. M. USSR Academy of Sciences. 1963. pp.74-85. 16. Murray J.L. Calculation of the Titanium-Aluminum Phase Diagram. //Metallurgical Transactions A. 1988. V.19A, p.243-247. 17. H. Okamoto. Ti Al. //J. Phase Equilibria. 1993. V.14, p.120. 18. Ogden H.R., Maykuth D.J., Finlay W.L., Jaffee R.I. Constitutions of Titanium-Aluminum Alloys. //Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. 1951. V. 191. p. 1150-1155. 19. Anderson C.D., Hofmeister W.H., Bayuzick R.J. Liquidus Temperatures in the Ti-Al System. //Metallugical Transactions A. 1993. V.24, p.61-66. 20. Kattner U.R., Lin J.C., Chang Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. //Metallurgical Transactions A. 1992. V.23, p.2081-2090. 21. Perepezko J.H. Phase Stability and Processing of Titanium Aluminides. //Proceedings of the International Symposium on Intermetallic Compounds, Structure and Mechanical Properties, (JIMIS-6). Sendai, Japan. 1991. p.239-243. 22. Perepezko J.H., Mishurda J.C. Phase Equilibria in the Titanium Aluminum System, //Titanium "92: Sci. and Technol.: Proc. Symp. 7th World Titanium Conf., San Diego, Calif., June 29 - July 2. 1992. V.l. Warrendale (Pa). 1992. p.563-570. 23. McCullough C., Valencia J.J., Levi C.G., Mehrabian R. Phase Equilibria and Solidification in Ti-Al Alloys. //Acta Metallurgies 1989. V.37, p. 1321-1336. 24. Chang J.Y., Moon I.G., Choi C.S. Microstructures of Heated Gamma(y)-Based Titanium-Aluminides. //J. Korean Inst. Met. & Mater. 1995. V.33. 11, p.1552-1561. 25. Collings E.W. Magnetic Studies of Phase Equilibria in Ti-Al (30 to 57 at.%) Alloys. //Metallurgical Transaction A. 1979. V.l OA. No. 4, p.463-473. 26. Jung I.S., Kim M.C., Lee J.H., Oh M.H., Wee D.M. Phase Equilibria of Ti-Al Alloy by Directional Solidification. //J. Cor. Inst. Met. & Mater. 1999. V.37. No. 4, p.448-453. 27. Jung I.S., Kim M.C., Lee J.H., Oh M.H., Wee D.M. High Temperature Phase Equilibria near Ti-50 at.% AI Composition in Ti-Al System Studied by Directional Solidification. //Intermetallics. 1999. V.7, p.1247-1253. 28. Okamoto H. Aluminum-Titanium. //J. Phase Equilibria. 2000. V. 21. No. 3, p. 311. 29. Zhang F., Chen S.L., Chang Y.A., Kattner U.R. A Themodynamic description of the Ti-Al system. //Intermetallics. 1997. V.5, p.471-482. 30. Kornilov I.I., Nartova T.T., Chernysheva S.P. About the phase diagram of Ti-Al in the titanium-rich part. //Izv. Academy of Sciences of the USSR. Metals. 1976. No. 6, p. 192-198. 31. Tsujimoto T., Adachi M. Reinvestigation of the Titanium Rich Region of the Titanium - Aluminum Equilibrium Diagram. //J. Institute of Metals. 1966. V.94. No. 10, p.358-363. 32. Van Loo F.J.J., Rieck G.D. Diffusion in the Titanium-Aluminum System II: Interdiffusion in the Composition Range between 25 and 100 at.% Ti. //Acta Metal. 1973. V.21, p.73-84. 33. Clark D., Jepson K.S., Lewis G.I. A Study of the Titanium -Aluminum System up to 40 at. % Aluminum. //J. Institute of Metals. 1962/63. V.91. No. 6, p. 197-203. 34. Sato T., Haung Y.C. The Equilibrium Diagram of the Ti-Al System. //Transactions of the Japan Institute of Metals. 1960. V.l, p.22-27. 35. Suzuki A., Takeyama M., Matsuo T. Transmission Electron Microscopy on the Phase Equilibria among ß, a and a2 Phases in Ti-Al Binary System. //Intermetallics. 2002. V.10, p.915-924. 36. Raman A., Schubert K. Uber den Aufbau Eunuger zu TiAb Verwandter Legierungsreihen. II. Untersuchungen in einigen Ti-Al-Si- und T4" 6 In-Systemen. HZ Metallkd. 1965. V.56, p.44-52. 37. Palm M., Zhang L.C., Stein F., Sauthoff G. Phase and Phase Equilibria in the Al-rich Part of the Al-Ti System above 900°C. //Intermetallics. 2002. V.10, p.523-540. 38. Schuster J.C., Ipser H. Phases and Phase Relations in the Partial System TiAh-TiAl. HZ. Metallkd. 1990. V.81, p.389-396. 39. Loiseau A., Vannffel C. TiAl2 a Reentrant Phase in the Ti AI System. //Phys. status solidi. 1988.V.l07. No. 2, p.655-671. 40. Hori S., Tai H., Matsumoto E. Solubility of titanium in aluminum in the solid state. //J. Japan Institute Light Metals. 1984. V.34. No. 7, p.377-381. 41. Abdel H.A., Allibert C.H., Durand F. Equilibrium between TiAh and Molten AI: Results from the Technique of Electromagnetic Phase Separation. //Z. Metallkd. 1984. V.75, p.455-458. 42. Minamino Y., Yamane T., Araki H., Takeuchi N., Kang Y., Miyamoto Y., Okamoto T. Solid Solubilities of Manganese and Titanium in Aluminum at 0.1 MPa and 2.1 Gpa. //Metallurgical Transactions A. 1991. V.22, p.783-786. 43. Liu Y.C., Yang G.C., Guo X.F., Huang J., Zhou Y.H. Coupled Growth Behavior in Rapidly Solidified Ti Al Peritectic Alloys. //J. Crystal Growth. 2001. V.222, p.645-654. 44. Mrowietz M., Weiss A. Solubility of Hydrogen in Titanium Alloys: I. The Solubility of Hydrogen in the System Tii-xGax, 0 45. Knapton A.G. The System Uranium-Titanium. //J. Institute of Metals. 1954/55. V.83, p.497-504. 46. Jamieson J.C. Crystal Structures of Titanium, Zirconium and Hafnium at High Pressures. //Science (Washington D.C.). 1963. V.140, p.72-73. 47. Sridharan S., Nowotny H. Studies in the Ternary System Ti-Ta-Al and in the Quaternary System Ti-Ta-Al-C. //Z. Metallkd. 1983. V.74, p.468-472. 48. Braun J., Ellner M. X-ray High-Temperature in-situ Investigation of the Aluminide TiAh (HfGa2 type). //J. Alloys and Compounds. 2000. V.309, p.l 18-122. 49. Braun J., Ellher M., Predel B. Zur Struktur der Hochtemperaturphase Ti-Al. //J. Alloys and Compounds. 1994. V.203, p.189-193. 50. Kumar K.S. X-Ray Peak Intensifies for the Binary Compound AljTi. //Powder Diffraction. 1990. V.5, p.165-167. 51. Bandyopadhyay J., Gupta K.P. Low Temperature Lattice Parameters of Al and Al Zn Alloys and Gruneisen Parameter of Al. //Cryogenics. 1978. V.l 8, p.54-55. 52. Kulikov I.S. Thermodynamics of carbides and nitrides. Chelyabinsk: Metallurgy, 1988.319p. 53. Peruzzi A., Abriata J.P. Al-Zr (Aluminum-Zirconium). //Binary Alloy Phase Diagrams, Second Edition Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V.l, p.241-243. 54. Murray J.L., McAlister A.J., Kahan D.J. The Al-Hf (Aluminum-Hafnium) System. //J. Phase Equilibria. 1998. No. 4, p.376-379. 55. Peruzzi A. Reinvestigation of the Zr-rich End of the Zr-Al Equilibrium Phase Diagram. //J. Nuclear Materials. 1992. V.186, p.89-99. 56. Sauders. N. Calculated Stable and Metastable Phase Equilibria in Al-Li-Zr Alloys. //Z. Metallkd. 1989. V.80, p.894-903. 57. Saunders N., Rivlin V.G. Thermodynamic Characterization of Al-Cr, Al-Zr, and Al-Cr-Zr Alloy Systems. //Materials Science and Technology. 1986. V.2, p.521-527. 58. Kaufman L., Nesor H. Calculation of the Ni-Al-W, Ni-Al-Hf and Ni-Cr-Hf Systems. //Canadian Metallurgical Quarterly. 1975. V.14, p.221-232. 59. Balducci G., Ciccioli A., Cigli G., Gozzi D., Anselmi-Tamburini U. Thermodynamic study of intermetallic phases in the Hf-Al system. //J. Alloys and Compounds. 1995. V.220, p. 117-121. 60. Matkovic P., Matkovic T., Vickovic I. Crystalline Structure of the Intermetallic Compound FeZr3. //Metallurgya. 1990. V.29, p.3-6. 61. Savitsky E.M., Tylkina M.A., Tsyganova I.A. Phase diagram of the zirconium - rhenium system. //Atomic Energy. 1959. V.7, pp. 724-727. 62. Ming L., Manghnani M.N., Katahara K.W. Investigation of a->x Transformation in the Zr-Hf System to 42 GPa, //J. Applied Physics. 1981. V.52, p.1332-1335. 63. Meng W.J., Faber J.jr., Okamoto P.R., Rehn L.E., Kestel B.J., Hitterman R.L. Neutron Diffraction and Transmission Electron Microscopy Study of Hydrogen-Induced Phase Transformations in Zr3Al. //J. Applied Physics. 1990. V.67, p.l 312-1319. 64. Clark N.J., Wu E. Hydrogen Absorption in the Zr-Al System. //J. Less-Common Metals. 1990. V. 163, p.227-243. 65. Nowotny H., Schob O., Benesovsky F. Die Kristallstruktur von Zr2Al und Hf2Al. //Monatshefte fur Chemie. 1961. V.92, p.1300-1303. 66. Nandedkar R.V., Delavignette P. On the Formation of a New Superstructure in the Zirconium-Aluminium System. //Physica Status Solidi A: Applied Research. 1982. V.73, p.K157-K160. 67. Kim S.J., Kematick R.J., Yi S.S., Franzen H.F. On the Stabilization of Zr5Al3 in the Mn5Si3-Type Structure by Interstitial Oxygen. //J. Less-Common Metals. 1988. V.137, p.55-59. 68. Kematick R.J., Franzen H.F. Thermodynamic Study of the Zirconium-Aluminum System. //J. Solid State Chemistry. 1984. V.54, p.226-234. 69. Hafez M., Slebarski A. Magnetic and Structural Investigations of Zri.xGdxAl2 Alloys. //J. Magnetism and Magnetic Materials. 1990. V.89, p. 124-128. 70. Desch P.B., Schwarz R.B., Nash P. Formation of Metastable Lb Phases in Al3Zr and Al-12.5% X-25% Zr(X=Li,Cr,Fe,Ni,Cu). //J. Less-Common Metals. 1991. V.168, p.69-80. 71. Ma Y., Romming C., Lebech V., Gjonnes J., Tafto J. Structure Refinement of Al3Zr using Single-Crystal X-ray Diffraction, Powder Neutron Diffraction and CBED. //Acta Crystallographica B. 1992. V.48, p. 11-16. 72. Schuster J.C., Nowotny H. Investigations of the Ternary Systems (Zr, Hf, Nb, Ta)-Al-C and Studies on Complex Carbides. //Z. Metallkd. 1980. V.71, p.341-346. 73. Maas J., Bastin G., Loo F.V., Metselaar R. The Texture in Diffusion-Grown Layers of . Trialuminides MeAl3 (Me=Ti, V, Ta, Nb, Zr, Hf) and VNi3. //Z Metallkd. 1983. V.74, p.294-299. 74. Wodniecki P., Wodniecka V., Kulinska A., Uhrmacher M., Lieb K.P. The Hafnium Aluminides HfAl3 and Н£гА13 Studied by Perturbed angular Corrlations with 181 Ta and mCd Probes. //J. Alloys and Compounds. 2000. V.312, p. 17-24. 75. Kuznetsov G.M., Barsukov A.D., Abas M.I. Study of the solubility of Mn, Cr, Ti and Zr in aluminum in the solid state. //Izv. universities Color Metallurgy. 1983. No. 1, pp. 96-100. 76. Rath V.V., Mohanty G.P., Mondolfo L.F. The Aluminum-Rich End of the Aluminum-Hafnium Diagram. //J. Institute of Metals. 1960/61. V.89, p.248-249. 77. Kattner U.R. AlNb. //Binary Alloy Phase Diagrams, second edition, Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990. V. 1, p. 179-181. 78. Suyama Ryuji, Kimura Masao, Hashimoto Keizo. Phase Stability and Fundamental Properties of Nb-Al Binary System. //Struct. Intermetallics. 1st Int. Symp. Struct. Intermetallics, Champion, Pa., Sept. 26-30, 1993, Warrendale (Pa). 1993. p.681-689. 79. Richards M.J. Contribution a l "etude du Systeme Niobiom-Aluminum. // Mémoires Scientifiques de la Revue de Metallurgie. 1964. V.61, p.265-270. 80. Herold A., Forsterling G., Kleinstuck K. Influence of the Real Structure on the Linear Thermal Expansion Coefficient of A15-type Intermetallic Compounds from Room Temperature to 10K. //Crystal Research and Technology. 1981. V. 16, p. 1137-1144. 81. Jorda J.L., Flukiger R., Muller J. A New Metallurgical Investigation of the Niobium-Aluminum System. //J. Less Common Metals. 1980. V.75, p.227-239. 82. Alfeu S.R., Carlos A.N. The Effect of Excess Aluminum on the Composition and Microstructure of Nb-Al Alloys Produced by Aluminothemic Reduction of Nb20s. //J. Materials Synthesis and Processing. 1999. V.7. No. 5, p.297-301. 83. Ahn I.S., Kim S.S., Park M.W., Lee K.M. Phase Characteristics of Mechanically Alloyed AI-10wt.%Nb Alloy. //J. Materials Science Letters. 2000. V.19, p.2015-2018. 84. Menon E.S.K., Subramanian P.R., Dimiduk D.M. Phase Transformations in Nb-Al-Ti Alloys. //Metallurgical Transaction A. 1996. V.27. No. 6, p. 1647-1659. 85. Kaufman L. Calculation of the Multicomponent Tantalum based Phase Diagrams. //CALPHAD. 1991. V. 15. No. 3, p.261-282. 86. Wriedt H.A. The Al-N (Aluminum-Nitrogen) System. //Bulletin of Alloy Phase Diagrams. 1986. V.7. No. 4, p.329-333. 87. Jones R.D., Rose K. Liquidus Calculations for III-IV Semiconductors. //CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry. 1984. V.8, p.343-354. 88. Hillert M., Josson S. An Assessment of the Al-Fe-N System. //Metallurgical Transaction A. 1992. V.23A, p.3141-3149. 89. Wriedt H.A., Murray J.L. N-Ti (Nitrogen-Titanium). //Binary Alloy Phase Diagrams, Second Edition, Ed. Massalski, ASM International, Materials Park, Ohio. 1990. V.3, p.2705-2708. 90. Zeng K., Schmid-Fetzer R. Critical Assessment and Thermodynamic Modeling of the Ti-N System. //Z. Metallkd. 1996.V.87. No. 7, p.540-554. 91. Etchessahar E., Bars J.P., Debuigne J. The Ti - N System: Equilibrium Between the Ô, e and a Phase and the Conditions of Formation of the Lobier and Marcon Metastable Phase. //J. Less-Common Metals. 1987. V.134, p. 123-139. 92. Vahlas C., Ladouce B.D., Chevalier P.Y., Bernard C., Vandenbukke L. A Thermodynamic Evaluation of the Ti N System. //Thermochemica Acta. 1991. V 180, p.23-37. 93. Etchessaher E., Sohn Y.U., Harmelin M., Debuigne J. The Ti N System: Kinetic, Calorimetric, Structure and Metallurgical Investigations of the ô-TiNo.si Phase. //J. Less-Common Metals. 1991. V. 167, p.261 -281. 94. Gusev A.I. Phase diagrams of ordered nonstoichiometric hafnium carbide and titanium nitride. //Reports of the Academy of Sciences. 1992. V.322. No. 5, pp. 918-923. 95. Gusev A.I., Rempel A.A. Phase diagrams of Ti C and Ti - N systems and atomic ordering of non-stoichiometric titanium carbide and nitride. //Reports of the Academy of Sciences. 1993. T.332. No. 6, pp. 717-721. 96. Lengauer W., Ettmayer P. Investigation of Phase Equilibria in the Ti N and Ti - Mo - N Systems. //Materials Science and Engineering A: Structure Materials: Properties, Microstructure and Processing. 1988. V.105/106. p.257-263. 97. Lengauer W. The Titanium Nitrogen System: A Study of Phase Reactions in the Subnitride Region by Means of Diffusion Couples. //Acta Metallurgica et Materialia. 1991. V.39, p.2985-2996. 98. Jonsson S. Assessment of the Ti N System. //Z. Metallkd. 1996.V.87. No. 9, p.691-702. 99. Ohtani H., Hillert M. A Thermodynamic Assessment of the Ti N System. //CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry. 1990. V.14, p.289-306. 100. Etchessahar E., Bars J.P., Debuigne J., Lamane A.P., Champin P. Titanium Nitrogen Phase Diagram and Diffusion Phenomena. //Titanium: Science and Technology Process 5 Int. Conf. Munich. Sept. 10-14 1984, V.3, Oberursel. 1985. p.1423-1430. 101. Wood F.W., Romans P.A., McCune R.A., Paasche O. Phases and Interdiffusion between Titanium and its Mononitride. //Rep. Infest. Bur. Mines. U.S. Dep. Inter. 1974. No. 7943. ii, p.40. 102. Em B.T., Latergaus I.S., Loryan V.E. Construction of the boundary of the region of existence of a solid solution of nitrogen in a-Ti using the neutron diffraction method. //Inorganic Mater. 1991. V.27. No. 3, pp. 517-520. 103. Kalmykov K.B., Rusina N.E., Dunaev S.F. Phase equilibria in the Al-Fe-Ni system at 1400K. //Vestn. Moscow Univ. Ser. 2. Chemistry. 1996. T.37. No. 5, pp. 469-473. 104. Toth L. Carbides and nitrides of transition materials. M.: Mir. 1974.294p. 105. Lengauer W. The Crystal Structure of ti-Ti3N2-x: An Additional New Phase in the Ti N System. //J. Less-Common Metals. 1996. V. 125, p. 127-134. 106. Christensen A.N., Alamo A., Landesman J.P. Structure of Vacancy-Ordered Titanium Heminitride 6"-Ti2N by Powder Neutron Diffraction. //Acta Crystallographica. Section C: Crystal Structure Communications. 1985. V.41, p.1009-1011. 107. Holmberg B. Structure Studies on the Titanium Nitrogen System. //Acta Chemica Scandinarica. 1962. V.16, p.1255-1261. 108. Lengauer W., Ettmayer P. The Crystal Structure of a New Phase in the Titanium-Nitrogen System. //J. Less-Common Metals. 1986. V.120, p.153-159. 109. Jiang C., Goto T., Hirai T. Non-stoichiometry of Titanium Nitride Plates Prepared by Chemical Vapor Deposition. //J. Alloys and Compounds. 1993. V.190, p. 197-200. 110. Eliot D.F., Glaser M., Ramakrishna V. Thermochemistry of steelmaking processes. M.: Metallurgy. 1969. 252 p. 111. Levinsky Yu.V. p-T State diagram of the zirconium-nitrogen system. //Physical chemistry. 1974. T.48, pp.486-488. 112. Domagala R.F., McPherson D.J., Hansen M. System Zirconium-Nitrogen. //Transaction of the American Institute of Mining, Metallurgical and Petroleum Engineering. 1956. V.206, p.98-105. 113. Massalski T.B. N-Zr. //Binary Alloy Phase Diagrams, Second Edition, Ed. T.B. Massalski, ASM International Materials Park, Ohio. 1990. V.3, p.2716-2717. 114. Ogawa T. Structural Stability and Thermodynamic Properties of Zr-N Alloys. //J. Alloys and Compounds. 1994. V.203, p.221-227. 115. Kosukhin V.B., Funke V.F., Minashkin V.L., Smirnov V.S., Efremov Yu.P. Preparation of coatings from zirconium nitride and carbonitride by CVD method. //Inorganic materials. News of the USSR Academies of Sciences. 1987. V.23, pp.52-56. 116. Lerch M., Fuglein E., Wrba J. Systhesis, Crystal Structure and High Temperature Behavior of Zr3N4. Z. Anorganische und Allgemeine Chemie. 1996. 622, p.367-372. 117. Massalski T.B. Hf-N. //Binary Alloy Phase Diagrams, Second Edition, Ed. T.B. Massalski, ASM Inter. Materials Park, Ohio. 1990*. V.2, p.2090-2092. 118. Christensen A.N. A Neutron Diffraction Investigation on Single Crystals of Titanium Oxide, Zirconium Carbide and Hafnium Nitride. //Acta Chemica Scandinavica. 1990. V.44, p.851-852. 119. Lengauer W., Rafaja D., Taubler R., Ettmayer P. Preparation of Binary Single-Phase Line Compounds via Diffusion Couples: The Subnitride Phase and C-Hf4N3.x. //Acta Metallurgica et Materialia. 1993. V.41, p.3505-3514. 120. Levinsky Yu.V. p-T State diagram of the niobium-nitrogen system. //Metals. 1974. V.1, pp. 52-55. 121. Huang W. Thermodynamic Properties of the Nb W - C - N System. //Z. Metallkd. 1997. V.88, p.63-68. 122. Lengauer W., Bohn M., Wollein V., Lisak K. Phase Reactions in the Nb N System Below 1400"C. //Acta Materialia. 2000. V.48, p.2633-2638. 123. Berger R., Lengauer W., Ettmayer P. The y-Nb4N3±x - 5-NbNi.x Phase Transition. //J. Alloys and Compounds. 1997. V.259, p.L9-L13. 124. Jogiet M., Lengauer W., Ettmayer P. III. Alloys and Compounds. 1998. V.46(2), p.233. 125. Huang W. Thermodynamic Assessment of the NbN System. //Metallurgical and Materials Transactions A. 1996. V.27A, p.3591-3600. 126. Balasubramanian K., Kirkaldy J.S. Experimental Investigation of the Thermodynamics of Fe-Nb-N austenite and Nonstoichiometric Niobium Nitride (1373-1673K). //Canadian Metallurgical Quarterly. 1989. V.28, p.301-315. 127. Christensen A.N. Preparation and Crystal Structure of ß-Nb2N and y-NbN. //Acta Chemica Scandinavica, A: Physical and Inorganic Chemistry. 1976. V.30, p.219-224. 128. Christensen A.N., Hazell R.G., Lehmann M.S. An X-ray and Neutron Diffraction Investigation of the Crystal Structure of y-NbN, //Acta Chemica Scandinavica, A: Physical and Inorganic Chemistry. 1981. V.35, p.l 11-115. 129. Lengauer W., Ettmayer P. Preparation and Properties of Compact Cubic 5-NbNi-x. //Monatshefte fur Chemie. 1986. V.l 17, p.275-286. 130. Yen C.M., Toth L.E., Shy Y.M., Anderson D.E., Rosner L.G. Superconducting Hc-Jc and Tc Measurements in the Nb-Ti-N, Nb-Hf-N and Nb-V-N Ternary Systems. //J. Applied Physics. 1967. V.38, p.2268-2271. 131. Terao N. New Phases of Niobium Nitride. //J. the Less-Common Metals. 1971. V.23, p.159-169. 132. Dobrynin A.B. New aluminum nitride ceramic materials. //Inorganic materials. 1992. V.28. No. 7, pp. 1349-1359. 133. Kulikov V.I., Mushkarenko Yu.N., Parkhomenko S.I., Prokhorov L.N. A new class of ceramic materials based on thermally conductive aluminum nitride. //Electronic equipment. Ser. Microwave Technology. 1993. T.2(456), pp.45-47. 134. Samsonov G.V. Nitrides. Kyiv: Naukova Dumka. 1969. 377 p. 135. Kral S., Lengauer W., Rafaja D., Ettmayer P. Critical Review on the Elastic Properties of Transition Metal Carbides, Nitrides and Carbonitrides. IIJ. Alloys and Compounds. 1998. V.265, p.215-233. 136. Samsonov G.V., Pilipenko A.T., Nazarchuk T.N. Analysis of refractory compounds. M: Metallurgizdat. 1962. 256 p. 137. Samonov G.V., Strashinskaya J1.B., Schiller E.A. Contact interaction of metal-like carbides, nitrides and borides with refractory metals at high temperatures. //Metallurgy and fuel. 1962. V.5, pp. 167-172. 138. Dai Ying, Nan Ce-wen. Synthesis of aluminum nitride whiskers via a vapor-liquid-solid process, //material Res. Soc. Symp. Proc. 1999. V.547, p.407-411. 139. Chen K.X., Li J.T., Xia Y.L., Ge C.C. Self-propagating high-temperature synthesis (SHS) and microstructure of aluminum nitride. //Int. J. Self-Propagating High-Temp. Synthesis. 1997. V.6(4), p.411-417. 140. Hwang C.C., Weng C.Y., Lee W.C., Chung S.L. Synthesis of A1N powder by a combustion synthesis method. //Int. J. Self-Propagating High-Temp. Synthesis. 1997. V.6(4), p.419-429. 141. Chung S.L., Yu W.L., Lin C.N. A self-propagating high-temperature synthesis method for synthesis of A1N powder. //J. Material Research. 1999. V.14(5), p. 1928-1933. 142. Ha H., Kim K.R., Lee H.C. A Study on the Synthesis of Titanium Nitride by SHS (Self-propagating High-temperature Synthesis) Method. //J. Cor. Ceramic. Soc. 1993. V.30. No. 12, p. 1096-1102. 143. Chen K., Ge C., Li J. Phase formation and thermodynamic analysis of self-propagating high-temperature synthesis Al-Zr-N system composites. //J. Material Research. 1998. V.13(9), p.2610-2613. 144. Chen K.X., Ge C.C., Li J.T. The effect of nitrogen pressure on in situ combustion synthesis of AIN-ZrN composites. //Metallurgical. Materials. Trans. A, 1999. V.30A(3A). p.825-828. 145. Garcia I., Olias J.S., Vazquez A.J. A new method for materials synthesis: solar energy concentrated by Fresnel lens. //J. Physics. 1999.IV. V.9. p.Pr3/435-Pr3/440. 146. Olias J.S., Garcia I., Vazquez A.J. Synthesis of TiN with solar energy condensed by a Fresnel lens. //J. Material Letters. 1999. V.38, p.379-385. 147. Boulmer-Leborgne C., Thomann A.L., Andreazza-Vignolle P., Hermann J., Craciun V., Echegut P., Crariun D. Excimer laser synthesis of A1N coating. //Appl. surface science. 1998. V. 125, p. 137-148. 148. Sicard E., Boulmer-Leborgne C., Sauvage T. Excimer laser induced surface nitriding of aluminum alloy. //Appl. Surface Science. 1998. V.127-129, p.726-730. 149. Boulmer-Leborgne C., Thomann A.L., Hermann J. Direct synthesis of metal nitride by laser. //NATO ASI Ser. 1996. Ser.E. V.307, p.629-636. 150. Thomann A.L., Sicard E., Boulmer-Leborgne C., Vivien C., Hermann J., Andreazza-Vignolle C., Andreazza P., Meneau C. Surface nitriding of titanium and aluminum by laser-induced plasma. //Surface Coating Technology. 1997.V.97. No.(1-3), p.448 452. 151. Dai X., Li Q., Ding M., Tian J. Thermodynamic aspect in synthesis of A1N powders by carbothermal reduction and nitridation process. //J. Material. Science. Technology. 1999. V.15(l), p.13-16. 152. Wang J., Wang W.L., Ding P.D., Yang Y.X., Fang L., Esteve J., Polo M.C., Sanchez G. Synthesis of cubic aluminum nitride by carbothermic nitridation reaction. //Diamond Relat. Mater. 1999. V.8(7), p. 1342-1344. 153. Pathak Lokesh Chandra, Ray Ajoy Kumar, Das Samar, Sivaramakrishnan C. S., Ramachandrarao P. Carbothermal synthesis of nanocrystalline aluminum nitride powders. //J. American Ceramic Society. 1999. V.82(l), p.257-260. 154. Clement F., Bastians P., Grange P. Novel low-temperature synthesis of titanium nitride: proposal for cyanonitridation mechanism. //Solid State Ionics. 1997. V.101-103. p.171-174. 155. Jung W.S., Ahn S.K. Synthesis of aluminum nitride by the reaction of aluminum sulfide with ammonia. //Materials Letters. 2000. V.43, p.53-56. 156. Hezler J., Leiberich R., Mick H.J., Roth P. Shock tube study of the formation of TiN molecules and particles. //Nanostruct. Materials. 1999. V.l 0(7), p. 1161-1171. 157. Uheda K., Takahashi M., Takizawa H., Endo T., Shimada M. Synthesis of aluminum nitride using urea-precursors. //Key Eng. Materials. 1999. V.l59-160, p.53-58. 158. Shimada S., Yoshimatsu M., Nagai H., Suzuku M., Komaki H. Preparation and properties of TiN and A1N films from alkoxide solution by thermal plasma CVD method. //Thin Solid Films. 2000. V.370, p.137-145. 159. Shimada S., Yoshimatsu M. Preparation of (Tii.xAlx)N films from mixed alkoxide solutions by plasma CVD. //Thin Solid Films. 2000. V.370, p.146-150. 160. Kim W.S., Sun H.N., Kim K.Y., Kim B.H. A study on the TiN Thin Film by Sol-Gel Method. //J. Cor. Ceramic. Soc. 1992. V.29. No. 4, p.328-334. 161. Sonoyama Noriyuki, Yasaki Yoichi, Sakata Tadayoshi. Formation of aluminum nitride using lithium nitride as a source of N3" in the molten aluminum chloride. //Chemical Letters. 1999. V.3, p.203-204. 162. Nakajima Kenichiro, Shimada Shiro. Electrochemical synthesis of TiN precursors and their conversion to fine particles. //J. Material Chem. 1998. V.8(4), p.955-959. 163. Pietzke M.A., Schuster J.C. Phase Equilibria of the Quaternary System Ti A1 - Sn - N at 900°C. //J. Alloys and Compounds. 1997. V.247, p. 198-201. 164. Schuster J.C., Bauer J. The Ternary System Titanium Aluminum - Nitrogen. //J. Solid State Chemistry. 1984. V.53, p.260-265. 165. Procopio A.T., El-Raghy T., Barsoum M.W. Synthesis of Ti4AlN3 and Phase Equilibria in the Ti - A1 N System. //Metallurgical and Materials Transactions A. 2000. V.31A, p.373-378. 166. Zeng K., Schmid-Fetzer R. Thermodynamic Modeling and Applications of the Ti A1 - N Phase Diagram. //Thermodynamics of alloy formation, 1997TMS annual meeting in Orlando, Florida, February 9-13. 1997. p.275-294. 167. Chen G., Sundman B. Thermodynamic Assessment of the Ti A1 - N System. //J. Phase Equilibria. 1998.V.19. No. 2, p.146-160. 168. Anderbouhr S., Gilles S., Blanquet E., Bernard C., Madar R. Thermodynamic Modeling of the Ti A1 - N System and Application to the Simulation of CVD Processes of the (Ti, A1)N Metastable Phase. //Chem.Vap.Deposition. 1999. V.5. No. 3, p.109-113. 169. Pietzka M.A., Schuster J.C. Phase Equilibria in the Quaternary System Ti A1 - C - N. //J. American Ceramic Society. 1996. V.79(9), p.2321-2330. 170. Lee H.D., Petuskey W.T. New Ternary Nitride in the Ti Al - N System. //J. American Ceramic Society. 1997. V.80. No. 3, p.604-608. 171. Ivanovskii A.L., Medvedeva N.I. Electronic Structure of Hexagonal Ti3AlC2 and Ti3AlN2. //Mendeleev Communications Electronic Version. 1999. V.l, p.36-38. 172. Barsoum M.W., Schuster J.C. Comment on "New Ternary Nitride in the Ti Al - N System". //J. American Ceramic Society. 1998.V.81. No. 3, p.785-789. 173. Barsoum M.W., Rawn C.J., El-Raghy T., Procopio A.T., Porter W.D., Wang H., Hubbard C.R. Thermal Properties of Ti4AlN3. //J. Applied Physics. 2000. V.87, p.8407-8414. 174. Procopio A.T., Barsoum M.W., El-Raghy T. Characterization of Ti4AlN3. //Metallurgical and Materials Transactions A. 2000. V.31A, p.333-337. 175. Myhra S., Crossley J.A.A., Barsoum M.W. Crystal-Chemistry of the Ti3AlN3 Layered Carbide/Nitride Phase-Characterization by XPS. III. Physics and Chemistry of Solids. 2001. V.62, p. 811-817. 176. El-Sayed M.H., Masaaki N., Schuster J.C. Interfacial Structure and Reaction Mechanism of AIN/Ti Joints. III. Materials Science. 1997. V.32, p.2715-2721. 177. Paransky Y., Berner A., Gotman I. Microstructure of Reaction Zone at the Ti A1N Interface. //Materials Letters. 1999. V.40, p. 180-186.9 178. Paransky Y.M., Berner A.I., Gotman I.Y., Gutmanas E.Y. Phase Recognition in A1N-Ti System by Energy Dispersive Spectroscopy and Electron Backscatter Diffraction. //Mikrochimica Acta. 2000. V.134, p.l71-177. 179. Gusev A.I. Phase equilibria in ternary systems M-X-X" and M-A1-X (M-transition metal, X, X" - B, C, N, Si) and crystal chemistry of ternary compounds. //Chemistry successes. 1996. V.65(5), pp.407-451. 180. Schuster J.C., Bauer J., Debuigne J. Investigation of Phase Equilibria Related to Fusion Reactor Materials: 1. The Ternary System Zr A1 - N. III. Nuclear Materials. 1983. V.116, p.131-135. 181. Schuster J.C. The Crystal Structure of Zr3AlN. //Z. Kristallography. 1986. V.175, p.211-215. 182. Schuster J.C., Bauer J. Investigation of Phase Equilibria Related to Fusion Reactor Materials: II. The Ternary System Hf-Al-N. III. Nuclear Materials. 1984. V.120, p.133-136. 183. Schuster J.C., Nowotny H. Phase Equilibria in the Ternary Systems Nb-Al-N and Ta-Al-N. //Z. Metallkd. 1985. V.76, p.728-729. 184. Jeitschko W., Nowotny H., Benesovsky F. Strukturchemische Unter Suchungen an Komplex -Carbiden und -Nitriden. //Monatsh Chem. 1964. V.95, p.l 56. 185. Reed S. Electron probe microanalysis. M.: Mir. 1979. 260 p. 186. Sokolovskaya E.M., Guzey JI.C. Metal chemistry. M.:Mosk. Univ. 1986. 264 p. 187. Abramycheva H.JI. Interaction of alloys based on iron, nickel and elements of groups IV–V with nitrogen at elevated partial pressure. Abstract of the candidate's dissertation, Moscow State University, 1999. 20 p. 188. Lupis K. Chemical thermodynamics of materials. M.: Metallurgy. 1989. 503 p. 189. Dinsdale A.T. SGTE Data for Pure Elements. //Calphad. 1991. V. 15. No. 4, p.317-425. 190. Kaufmann L., Nesor H. Coupled Phase Diagrams and Thermochemical Data for Transition Metal Binary Systems V. // Calphad. 1978. V.2. No. 4, p.325-348. 191. Voronin G.F. Partial thermodynamic functions of heterogeneous mixtures and their application in the thermodynamics of alloys. //In the book: Modern problems of physical chemistry. M.: Moscow. Univ. 1976. vol.9. p.29-48. 192. Kaufman L., Bershtein X. Calculation of state diagrams using a computer: Transl. from English M.: Mir. 1972. 326 p. 193. Belov G.V., Zaitsev A.I. Using the Monte Carlo method to determine the phase composition of heterogeneous systems. // Abstracts of the XIV International Conference on Chemical Thermodynamics. St. Petersburg: Scientific Research Institute of St. Petersburg State University. T.2002. p.317-318. 194. Khan Yu.S., Kalmykov K.B., Dunaev S.F., Zaitsev A.I. Phase equilibria in the Ti-Al-N system at 1273 K. // Reports of the Academy of Sciences. 2004. t.396. No. 6, pp. 788-792. 195. Han Y.S., Kalmykov K.V., Dunaev S.F., Zaitsev A.I. Solid-State Phase Equilibria in the Titanium-Aluminum-Nitrogen System. //J. Phase Equilibria and Diffusion. 2004. V.25. No. 5, p.427-436. 196. State diagrams of binary metal systems. Directory: In 3 volumes: T.Z. Book 1 /Under. General Ed. N.P. Lyakisheva. M.: Mechanical engineering. 1999. 880 p. 197. Wang T., Jin Z., Zhao J.C. Thermodynamic Assessment of the Al-Zr Binary System. //J. Phase Equilibria. 2001. V.22. No. 5, p.544-551. 198. Turkdogan E.T. Physical chemistry of high-temperature processes. M.: Metallurgy. 1985. 344 p. 199. Han Y.S., Kalmykov K.V., Abramycheva N.L., Dunaev S.F. Structure of the Al-Zr-N system at 1273K and 5Mpa. //VIII International conference of crystalchemistry of intermetallic compounds. Lviv. Ukraine. September 25-28.2002. p.65. 200. Khan Yu.S., Kalmykov K.B., Zaitsev A.I., Dunaev S.F. Phase equilibria in the Zr-Al-N system at 1273 K. //Metals. 2004. T.5, pp.54-63. 201. Han Yu Sin, Kalmykov K.B., Dunaev S.F. Interaction of aluminum nitride with elements of group IVB. //International Conference of Undergraduate and Postgraduate Students on Basic Sciences "Lomonosov-2003". April 15-18, 2003 section Chemistry. T.2, p.244. Please note that the scientific texts presented above are posted for informational purposes only and were obtained through original dissertation text recognition (OCR). Therefore, they may contain errors associated with imperfect recognition algorithms. There are no such errors in the PDF files of dissertations and abstracts that we deliver. At the end of the 90s, the 7th edition of the Electrical Installation Rules (PUE) was introduced in Russia, according to which the electrical installation of internal networks of buildings from aluminum cables and wires with a cross-section of less than 16 mm2 is prohibited, and it is prescribed that they be made from copper wire. The reason for the change in regulatory requirements was due to certain properties of aluminum. Aluminum cables and wires have long been widely used both for wiring internal power networks in buildings for various purposes, and for laying external power lines. This is due to the following properties of aluminum: However, other features of aluminum are: high fluidity, which does not provide sufficient quality of contacts for a long time; low strength under mechanical stress on fracture; low heat resistance, leading to increased fragility when overheated - led to the introduction of a ban on the electrical installation of small cross-section aluminum wires for internal power supply networks. One of the main reasons that influenced the change in PUE requirements is that during operation, a thin oxide film is formed on the surface of aluminum wires, which has much worse electrical conductivity than the base metal. As a result, a higher contact resistance is formed at the junction of the wires, which significantly increases the possibility of heating the contacts and the risk of their destruction and fire. Copper, used as a material for electrical cables and wires, despite its higher cost, does not have the listed disadvantages of aluminum and has a number of advantages: higher conductivity; does not form an oxide film on the surface; higher flexibility, this allows the production of wires with a very small cross-section of up to 0.3 mm2, which cannot be made from aluminum. Since many old buildings still have electrical networks made of aluminum wires, during renovation there is often a need to connect wiring made of different materials - copper and aluminum. According to the same Electrical Installation Rules, the connection of aluminum and copper wires can be made in several ways: The article was prepared based on materials from the site http://energy-systems.ru/ Lesson objectives: consider the distribution of aluminum in nature, its physical and chemical properties, as well as the properties of the compounds it forms. Progress 2. Studying new material. Aluminum The main subgroup of group III of the periodic table consists of boron (B), aluminum (Al), gallium (Ga), indium (In) and thallium (Tl). As can be seen from the above data, all these elements were discovered in the 19th century. Discovery of metals of the main subgroup
III
groups
1806 1825 1875 1863 1861 G. Lussac, G.H. Ørsted L. de Boisbaudran F. Reich, W. Crooks L. Tenard (Denmark) (France) I.Richter (England) (France) (Germany) Boron is a non-metal. Aluminum is a transition metal, while gallium, indium and thallium are full-fledged metals. Thus, with increasing radii of the atoms of the elements of each group of the periodic table, the metallic properties of simple substances increase. In this lecture we will take a closer look at the properties of aluminum. MUNICIPAL BUDGET EDUCATIONAL INSTITUTION GENERAL EDUCATION SCHOOL No. 81 Aluminum. The position of aluminum in the periodic table and the structure of its atom. Being in nature. Physical and chemical properties of aluminum. chemistry teacher MBOU secondary school No. 81 2013 Lesson topic: Aluminum. The position of aluminum in the periodic table and the structure of its atom. Being in nature. Physical and chemical properties of aluminum. Lesson objectives: consider the distribution of aluminum in nature, its physical and chemical properties, as well as the properties of the compounds it forms. Progress 1. Organizational moment of the lesson. 2. Studying new material. Aluminum The main subgroup of group III of the periodic table consists of boron (B),aluminum
(Al), gallium (Ga), indium (In) and thallium (Tl). As can be seen from the above data, all these elements were discovered in the 19th century. Discovery of metals of the main subgroup of group III 1806 1825 1875 1863 1861 G. Lussac, G.H. Ørsted L. de Boisbaudran F. Reich, W. Crooks L. Tenard (Denmark) (France) I.Richter (England) (France) (Germany) Boron is a non-metal. Aluminum is a transition metal, while gallium, indium and thallium are full-fledged metals. Thus, with increasing radii of the atoms of the elements of each group of the periodic table, the metallic properties of simple substances increase. In this lecture we will take a closer look at the properties of aluminum. 1.
The position of aluminum in D. I. Mendeleev’s table. Atomic structure, exhibited oxidation states. The element aluminum is located in group III, the main “A” subgroup, period 3 of the periodic system, serial number No. 13, relative atomic mass Ar(Al) = 27. Its neighbor on the left in the table is magnesium - a typical metal, and on the right is silicon - a non-metal . Consequently, aluminum must exhibit properties of some intermediate nature and its compounds are amphoteric. Al +13) 2 ) 8 ) 3 , p – element, Ground state 1s 2 2s 2 2p 6 3s 2 3p 1 Excited state 1s 2 2s 2 2p 6 3s 1 3p 2 Aluminum exhibits an oxidation state of +3 in compounds: Al 0 – 3 e - → Al +3 2. Physical properties Aluminum in its free form is a silvery-white metal with high thermal and electrical conductivity. Melting point 650 O C. Aluminum has a low density (2.7 g/cm 3
) - approximately three times less than that of iron or copper, and at the same time it is a durable metal. 3. Being in nature In terms of prevalence in nature, it ranks1st among metals and 3rd among elements, second only to oxygen and silicon. The percentage of aluminum content in the earth's crust, according to various researchers, ranges from 7.45 to 8.14% of the mass of the earth's crust. In nature, aluminum occurs only in compounds(minerals). Some of them: Bauxite - Al 2 O 3 H 2 O (with impurities of SiO 2, Fe 2 O 3, CaCO 3) Nephelines - KNa 3 4 Alunites - KAl(SO 4 ) 2 2Al(OH) 3 Alumina (mixtures of kaolins with sand SiO 2, limestone CaCO 3, magnesite MgCO 3) Corundum - Al 2 O 3 Feldspar (orthoclase) - K 2 O×Al 2 O 3 ×6SiO 2 Kaolinite - Al 2 O 3 × 2SiO 2 × 2H 2 O Alunite - (Na,K) 2 SO 4 ×Al 2 (SO 4) 3 ×4Al(OH) 3 Beryl - 3BeO Al 2 O 3 6SiO 2 Bauxite Al2O3 Corundum Ruby Sapphire 4. Chemical properties of aluminum and its compounds Aluminum reacts easily with oxygen under normal conditions and is coated with an oxide film (which gives it a matte appearance). Its thickness is 0.00001 mm, but thanks to it, aluminum does not corrode. To study the chemical properties of aluminum, the oxide film is removed. (Using sandpaper, or chemically: first dipping it into an alkali solution to remove the oxide film, and then into a solution of mercury salts to form an alloy of aluminum with mercury - amalgam). I. Interaction with simple substances Already at room temperature, aluminum actively reacts with all halogens, forming halides. When heated, it reacts with sulfur (200 °C), nitrogen (800 °C), phosphorus (500 °C) and carbon (2000 °C), with iodine in the presence of a catalyst - water: 2Al + 3S = Al 2 S 3 (aluminum sulfide), 2Al + N 2 = 2AlN (aluminum nitride), Al + P = AlP (aluminum phosphide), 4Al + 3C = Al 4 C 3 (aluminum carbide). 2 Al + 3 I 2 = 2 AlI 3 (aluminum iodide) All these compounds are completely hydrolyzed to form aluminum hydroxide and, accordingly, hydrogen sulfide, ammonia, phosphine and methane: Al 2 S 3 + 6H 2 O = 2Al(OH) 3 + 3H 2 S Al 4 C 3 + 12H 2 O = 4Al(OH) 3 + 3CH 4 In the form of shavings or powder, it burns brightly in air, releasing a large amount of heat: 4Al + 3O 2 = 2Al 2 O 3 + 1676 kJ. II. Interaction with complex substances Interaction with water:
2 Al + 6 H 2 O = 2 Al (OH) 3 + 3 H 2 without oxide film Interaction with metal oxides: Aluminum is a good reducing agent, as it is one of the active metals. It ranks in the activity series immediately after the alkaline earth metals. That's whyrestores metals from their oxides. This reaction, aluminothermy, is used to produce pure rare metals, such as tungsten, vanadium, etc. 3 Fe 3 O 4 + 8 Al = 4 Al 2 O 3 + 9 Fe +Q Thermite mixture Fe 3 O 4 and Al (powder) – also used in thermite welding. Сr 2 О 3 + 2Аl = 2Сr + Al 2 О 3 Interaction with acids:
With sulfuric acid solution: 2 Al + 3 H 2 SO 4 = Al 2 (SO 4 ) 3 + 3 H 2 It does not react with cold concentrated sulfur and nitrogen (passivates). Therefore, nitric acid is transported in aluminum tanks. When heated, aluminum is able to reduce these acids without releasing hydrogen: 2Al + 6H 2 SO 4 (conc) = Al 2 (SO 4 ) 3 + 3SO 2 + 6H 2 O, Al + 6HNO 3(conc) = Al(NO 3) 3 + 3NO 2 + 3H 2 O. Interaction with alkalis.
2 Al + 2 NaOH + 6 H 2 O = 2 NaAl(OH) 4 + 3 H 2 Na[Al(OH) 4 ] – sodium tetrahydroxyaluminate At the suggestion of the chemist Gorbov, during the Russo-Japanese War this reaction was used to produce hydrogen for balloons. With salt solutions: 2Al + 3CuSO 4 = Al 2 (SO 4 ) 3 + 3Cu If the surface of aluminum is rubbed with mercury salt, the following reaction occurs: 2Al + 3HgCl 2 = 2AlCl 3 + 3Hg The released mercury dissolves the aluminum, forming an amalgam. 5. Application of aluminum and its compounds The physical and chemical properties of aluminum have led to its widespread use in technology.The aviation industry is a major consumer of aluminum: 2/3 of the aircraft consists of aluminum and its alloys. A steel plane would be too heavy and could carry far fewer passengers.That's why aluminum is called a winged metal.Cables and wires are made from aluminum: with the same electrical conductivity, their mass is 2 times less than the corresponding copper products. Considering the corrosion resistance of aluminum, it ismanufacture machine parts and containers for nitric acid. Aluminum powder is the basis for the production of silver paint to protect iron products from corrosion, and to reflect heat rays, such paint is used to cover oil storage tanks and firefighter suits. Aluminum oxide is used to produce aluminum and also as a refractory material. Aluminum hydroxide is the main component of the well-known drugs Maalox and Almagel, which reduce the acidity of gastric juice. Aluminum salts are highly hydrolyzed. This property is used in the process of water purification. Aluminum sulfate and a small amount of slaked lime are added to the water to be purified to neutralize the resulting acid. As a result, a voluminous precipitate of aluminum hydroxide is released, which, settling, carries with it suspended particles of turbidity and bacteria. Thus, aluminum sulfate is a coagulant. 6. Aluminum production 1) A modern, cost-effective method for producing aluminum was invented by the American Hall and the Frenchman Héroult in 1886. It involves electrolysis of a solution of aluminum oxide in molten cryolite. Molten cryolite Na 3 AlF 6 dissolves Al 2 O 3, How water dissolves sugar. Electrolysis of a “solution” of aluminum oxide in molten cryolite occurs as if the cryolite were only the solvent and the aluminum oxide the electrolyte. 2Al 2 O 3 electric current → 4Al + 3O 2 In the English “Encyclopedia for Boys and Girls,” an article on aluminum begins with the following words: “On February 23, 1886, a new metal age began in the history of civilization - the age of aluminum. On this day, Charles Hall, a 22-year-old chemist, walked into his first teacher's laboratory with a dozen small balls of silvery-white aluminum in his hand and with the news that he had found a way to make the metal cheaply and in large quantities." So Hall became the founder of the American aluminum industry and an Anglo-Saxon national hero, as a man who turned science into a great business. 2) 2Al 2 O 3 + 3 C = 4 Al + 3 CO 2 THIS IS INTERESTING: 3. Consolidation of the studied material No. 1. To obtain aluminum from aluminum chloride, calcium metal can be used as a reducing agent. Write an equation for this chemical reaction and characterize this process using an electronic balance. No. 2. Complete the equations of chemical reactions: No. 3. Solve the problem: 4. Homework Slide 2 AL Element III (A) of table group D.I. Mendeleev Element with serial number 13, its Element of the 3rd period The third most common name in the earth's crust is derived from the Latin. "Aluminis" – alum Danish physicist Hans Oersted (1777-1851) Aluminum was first obtained by him in 1825 by the action of potassium amalgam on aluminum chloride followed by distillation of mercury. Modern production of aluminum The modern production method was developed independently of each other: by the American Charles Hall and the Frenchman Paul Héroux in 1886. It consists of dissolving aluminum oxide in molten cryolite, followed by electrolysis using consumable coke or graphite electrodes. As a student at Oberlin College, he learned that he could become rich and gain the gratitude of humanity if he could invent a way to produce aluminum on an industrial scale. Like a man possessed, Charles experimented with producing aluminum by electrolysis of cryolite-alumina melt. On February 23, 1886, a year after graduating from college, Charles produced the first aluminum using electrolysis. Charles Hall (1863 – 1914) American chemical engineer Paul Héroux (1863-1914) - French chemical engineer In 1889, he opened an aluminum plant in Front (France), becoming its director, he designed an electric arc furnace for steel smelting, named after him; he also developed an electrolytic method for producing aluminum alloys 8 Aluminum 1. From the history of discovery Home Next During the period of discovery of aluminum, the metal was more expensive than gold. The British wanted to honor the great Russian chemist D.I. Mendeleev with a rich gift; they gave him chemical scales, in which one cup was made of gold, the other of aluminum. An aluminum cup has become more expensive than a gold one. The resulting “silver from clay” interested not only scientists, but also industrialists and even the Emperor of France. Further 9 Aluminum 7. Contents in the earth’s crust main Next Found in nature The most important mineral of aluminum today is bauxite. The main chemical component of bauxite is alumina (Al 2 O 3) (28 - 80%). 11 Aluminum 4. Physical properties Color – silver-white t pl. = 660 °C. t kip. ≈ 2450 °C. Electrically conductive, thermally conductive Lightweight, density ρ = 2.6989 g/cm 3 Soft, plastic. home Next 12 Aluminum 7. Occurrence in nature Bauxite – Al 2 O 3 Alumina – Al 2 O 3 main Next 13 Aluminum main Fill in the missing words Aluminum is an element of group III, the main subgroup. The charge of the nucleus of an aluminum atom is +13. There are 13 protons in the nucleus of an aluminum atom. There are 14 neutrons in the nucleus of an aluminum atom. There are 13 electrons in an aluminum atom. The aluminum atom has 3 energy levels. The electron shell has the structure 2e, 8e, 3e. At the outer level there are 3 electrons in an atom. The oxidation state of an atom in compounds is +3. The simple substance aluminum is a metal. Aluminum oxide and hydroxide are amphoteric in nature. Further 14 Aluminum 3 . Structure of a simple substance Metal Bond - metal Crystal lattice - metal, cubic face-centered main Next 15 Aluminum 2. Electronic structure 27 A l +13 0 2e 8e 3e P + = 13 n 0 = 14 e - = 13 1 s 2 2 s 2 2p 6 3s 2 3p 1 Brief electronic notation 1 s 2 2 s 2 2p 6 3s 2 3p 1 Filling order home Next 16 Aluminum 6. Chemical properties 4A l + 3O 2 = 2Al 2 O 3 t 2Al + 3S = Al 2 S 3 C non-metals (with oxygen, with sulfur) 2 A l + 3Cl 2 = 2AlCl 3 4Al + 3C = Al 4 C 3 C non-metals (with halogens, with carbon) (Remove oxide film) 2 Al + 6 H 2 O = 2Al(OH) 2 + H 2 C water 2 Al + 6 HCl = 2AlCl 3 + H 2 2Al + 3H 2 SO 4 = Al 2 (SO 4) 3 + H 2 C acids and 2 Al + 6NaOH + 6H 2 O = 2Na 3 [ Al (OH ) 6 ] + 3H 2 2Al + 2NaOH + 2H 2 O = 2NaAlO 2 + 3H 2 C alkalis and 8Al + 3Fe 3 O 4 = 4Al 2 O 3 + 9Fe 2Al + WO 3 = Al 2 O 3 + W C o x i d a m m e t a l l o v home Next 17 Aluminum 8. Preparation 1825 H. Oersted: AlCl 3 + 3K = 3KCl + Al: Electrolysis (t pl. = 2050 ° C): 2Al 2 O 3 = 4 Al + 3O 2 Electrolysis (in molten cryolite Na 3 AlF 6, t pl. ≈ 1000 ° C): 2Al 2 O 3 = 4 Al + 3O 2 main NextRecommended list of dissertations
Interaction of alloys based on iron, nickel and elements of groups IV-VI with nitrogen at elevated partial pressure 1999, Candidate of Chemical Sciences Abramycheva, Natalya Leonidovna
Phase equilibria in M-M"-N systems at elevated pressure 2001, Candidate of Chemical Sciences Vyunitsky, Ivan Viktorovich
Decomposition of zirconium-niobium carbide solid solutions and segregation of the ZrC phase in the ternary system Zr - Nb - C 2002, candidate of physical and mathematical sciences Rempel, Svetlana Vasilievna
Modeling of internal nitriding processes of heat-resistant steels and alloys 2001, Doctor of Technical Sciences Petrova, Larisa Georgievna
Interaction of elements in compositions of refractory metals with heat-resistant alloys based on nickel and iron 1999, Candidate of Chemical Sciences Kerimov, Elshat Yusifovich
Introduction of the dissertation (part of the abstract) on the topic “Phase equilibria in systems nitrogen-aluminum-transition metal of groups IV-V”
Similar dissertations in the specialty "Physics of Condensed Matter", 04/01/07 code VAK
Phase equilibria and directed synthesis of solid solutions in ternary semiconductor systems with two volatile components 1998, Doctor of Chemical Sciences Semenova, Galina Vladimirovna
Quasicrystalline phases in Al-Mn-Si, Al-Cu-Fe, Al-Cu-Co systems: conditions of existence, structure, properties 2012, Candidate of Chemical Sciences Kazennov, Nikita Vladimirovich
Calculation of multicomponent phase diagrams and their use for the development of alloys and improvement of their processing technology 2001, Doctor of Technical Sciences Smagulov, Dauletkhan Uyalovich
Synthesis of nitrides of elements of groups III-VI and composite materials based on them by nitriding of ferroalloys in combustion mode 2009, Doctor of Technical Sciences Chukhlomina, Lyudmila Nikolaevna
Thermodynamics of phase equilibria in metal alloys containing carbon 2001, Candidate of Chemical Sciences Kachurina, Olga Ivanovna
Conclusion of the dissertation on the topic “Physics of Condensed Matter”, Han Yu Xing
List of references for dissertation research Candidate of Physical and Mathematical Sciences Han Yu Xing, 2004
Aluminum as an electrical conductor
Connection of aluminum and copper wires
Download:
Preview:
Think! Why can't this reaction be carried out in an aqueous solution?
Al+H 2 SO 4 (solution) ->
Al + CuCl 2
->
Al + HNO 3 (conc) - t ->
Al + NaOH + H 2 O ->
An aluminum-copper alloy was exposed to an excess of concentrated sodium hydroxide solution while heating. 2.24 liters of gas (n.o.) were released. Calculate the percentage composition of the alloy if its total mass was 10 g?