Enzymes, Enzyme-Substrate Complex and Activation Energy
The most important function of proteins is catalytic; it is performed by a certain class of proteins - enzymes. More than 2000 enzymes have been identified in the body. Enzymes are biological catalysts of a protein nature that significantly accelerate biochemical reactions. Thus, the enzymatic reaction occurs 100-1000 times faster than without enzymes. They differ in many properties from catalysts used in chemistry. Enzymes speed up reactions under normal conditions, unlike chemical catalysts.
In humans and animals, a complex sequence of reactions occurs in a few seconds, which require a long time (days, weeks or even months) to complete using conventional chemical catalysts. Unlike reactions without enzymes, by-products are not formed in enzymatic reactions (the yield of the final product is almost 100%). During the transformation process, enzymes are not destroyed, so a small amount of them can catalyze chemical reactions of a large number of substances. All enzymes are proteins and have properties characteristic of them (sensitivity to changes in the pH of the environment, denaturation at high temperatures, etc.).
Enzymes are divided according to their chemical nature into one-component (simple) And two-component (complex) .
One-component (simple)
Single-component enzymes consist only of proteins. The simple ones mainly include enzymes that carry out hydrolysis reactions (pepsin, trypsin, amylase, papain, etc.).
Two-component (complex)
Unlike simple enzymes, complex enzymes contain a non-protein part - a low molecular weight component. The protein part is called apoenzyme (enzyme carrier), non-protein – coenzyme (active or prosthetic group). The non-protein part of enzymes can be represented either by organic substances (for example, derivatives of vitamins, NAD, NADP, uridine, cytidyl nucleotides, flavins), or inorganic (for example, metal atoms - iron, magnesium, cobalt, copper, zinc, molybdenum, etc. .).
Not all necessary coenzymes can be synthesized by organisms and therefore must be supplied with food. The lack of vitamins in human and animal food causes the loss or decrease in the activity of the enzymes that contain them. Unlike the protein part, organic and inorganic coenzymes are very resistant to unfavorable conditions (high or low temperatures, radiation, etc.) and can be separated from the apoenzyme.
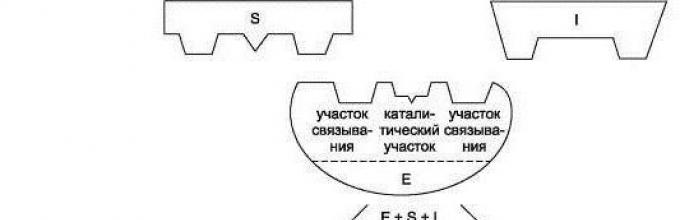
Enzymes are characterized by high specificity: they can convert only appropriate substrates and catalyze only certain reactions of one type. Its protein component determines, but not its entire molecule, but only a small section of it - active center . Its structure corresponds to the chemical structure of the substances that react. Enzymes are characterized by a spatial correspondence between the substrate and the active site. They fit together like a key in a lock. There can be several active centers in one enzyme molecule. Not only enzymes, but also some other proteins (heme in the active centers of myoglobin and hemoglobin) have an active center, that is, a place of connection with other molecules. Enzymatic reactions occur in successive stages - from several to dozens.
The activity of complex enzymes appears only when the protein part is combined with the non-protein part. Also, their activity manifests itself only under certain conditions: temperature, pressure, pH of the environment, etc. Enzymes of different organisms are most active at the temperature to which these creatures are adapted.
Enzyme-substrate complex
The bonds between the substrate and the enzyme form enzyme-substrate complex.
At the same time, it changes not only its own conformation, but also the conformation of the substrate. Enzymatic reactions can be inhibited by their own reaction products - when products accumulate, the reaction rate decreases. If there are few reaction products, the enzyme is activated.
Substances that penetrate into the region of the active center and block the catalytic groups of enzymes are called inhibitors (from lat. inhibere- hold back, stop). Enzyme activity is reduced by heavy metal ions (lead, mercury, etc.).
Enzymes reduce activation energy, which is the level of energy required to make molecules react.
Activation energy
Activation energy - this is the energy that is spent on breaking a certain bond for the chemical interaction of two compounds. Enzymes have a specific location in the cell and the body as a whole. In a cell, enzymes are contained in certain parts of the cell. Many of them are associated with the membranes of cells or individual organelles: mitochondria, plastids, etc.
Organisms are able to regulate the biosynthesis of enzymes. This makes it possible to maintain a relatively constant composition despite significant changes in environmental conditions and to partially modify enzymes in response to such changes. The effect of various biologically active substances - hormones, drugs, plant growth stimulants, poisons, etc. - is that they can stimulate or suppress one or another enzymatic process.
Some enzymes take part in the active transport of substances across membranes.
The names of most enzymes are characterized by the suffix -az-. It is added to the name of the substrate with which the enzyme interacts. For example, hydrolases – catalyze reactions of the splitting of complex compounds into monomers due to the addition of a water molecule at the site of breaking the chemical bond in the molecules of proteins, polysaccharides, and fats; oxide reductase – accelerate redox reactions (transfer of electrons or protons); isomerases– promote internal molecular rearrangement (isomerization), transformation of isomers, etc.
As one student said during an exam in biological chemistry, “an enzyme is when there is very little of something, but it can move a mountain.” The student was not praised for such a definition, but he grasped the essence of things correctly - enzymes accelerate many times the reaction between substances, which without them either does not occur at all or occurs many times more slowly.
The first enzyme with a lytic effect on the cell walls of bacteria was lysozyme, discovered in 1922 by the famous English microbiologist Alexander Fleming, who first discovered lysozyme in many tissues and secretions of animal organisms and suggested that it is a protective agent that allows higher organisms to fight existing in their habitat by pathogenic bacteria. It is interesting to note that this discovery of Fleming was made almost 10 years earlier than his second, more famous scientific achievement - the discovery and deciphering of the structure (together with H.W. Florey and E.B. Cheyne) of penicillin, the first and most widely used antibiotic. The first reports that bacteria have the ability to produce bacteriolytic enzymes began to appear already in the early 50s. To date, they have been found in all studied bacteria.
In this case, the enzyme, compared to the amount of interacting substances, may be negligibly small - millions of times less in weight. And, what is especially interesting, the enzyme does not decrease in quantity: one of its molecules can serve the reaction many times and be ready for work again (this is how a good mill will grind new grains again and again without noticeable wear of the millstones).
This means that these special compounds are classified as catalysts - accelerators of chemical reactions. Such catalysts are also known in inanimate nature, therefore those that are found only in living beings are called biocatalysts. They are proteins in composition. Each reaction requires its own enzyme: it fits it like a key to a lock. Therefore, there are a great many such catalyst proteins in plants, animals and microbes.
So far, however, the mechanism by which the reaction occurs has not yet been fully studied. Some scientists believe that large enzyme molecules are a kind of meeting ground for the molecules of substances involved in the reaction. There are other assumptions. We will not analyze them, but will draw a general conclusion: for the life of any organism, many enzymes are needed, and their set will also determine the method of nutrition of the microorganism.
Those microbes that require only inorganic compounds for nutrition - oxygen, solutions of certain salts, sulfur - are called autotrophs (translated into Russian this name means: those who feed themselves).
Autotrophs build their proteins and nucleic acids, one might say, from nothing - from nitrogenous compounds, water, oxygen... It’s like building the Cheops pyramid from tiny bricks (and not from the multi-ton stone blocks from which it is actually composed ). You can even calculate how many times the entire “pyramid” (say, a huge molecule of deoxyribonucleic acid - and what a long name!) is larger than one “brick” - a molecule of carbon dioxide or ammonia.
The molecular weight of deoxyribonucleic acid (usually abbreviated as DNA) in bacteria is 1000 million; the molecular weight of carbon dioxide is 44. This means that the entire “building” is 20 million times larger than a “brick” (it may be recalled that about 100 thousand bricks are now spent on a multi-storey building). And the entire molecular structure is “built” in just half an hour (the period required for a bacterial cell to produce its own kind).
If we talk about the location of bacteriolytic enzymes in a bacterial culture, then we should first of all divide them in this regard into three groups.
The first group consists of autolysins - bacteriolytic enzymes that are always present (in an active or inactive state) in the cell wall itself. They take part in the process of growth and differentiation of bacterial cells. In a bacterial cell, apparently, there is normally a relationship between the activities of enzymes that destroy and synthesize components of the cell wall. Indeed, the incorporation of newly synthesized materials into the cell wall cannot occur without the preliminary cleavage of certain chemical bonds.
The processes of lysis and biosynthesis of the wall occur simultaneously with the growth and development of the bacterial cell, and only in the later stages of development, when the biosynthetic processes subside and the activity of lytic enzymes remains at the same level, does lysis of the bacterial cell occur.
The second group includes lytic enzymes of bacterial spores. They are activated along with other enzymes involved in the degradation of biopolymers during the period of sporulation (spore formation) and during the germination of bacterial spores. These enzymes take part in the processes of destruction of the membrane and as autolysins in the processes of growth and morphogenesis of the bacterial cell.
Finally, the third group is extracellular lytic enzymes. Their biological role apparently lies in the fact that bacteria that synthesize and secrete such enzymes into the environment have an advantage over other bacteria, primarily in food sources. By destroying the cells of other bacteria, the bacterium that produces lytic enzymes uses amino acids, carbohydrates and other components of the lysed cell for its own needs. In addition, this group of bacteriolytic enzymes plays an absolutely important role in protecting cells that secrete these enzymes into the environment from other bacteria living in the same ecological niche.
Millions of chemical reactions take place in the cell of any living organism. Each of them is of great importance, so it is important to maintain the speed of biological processes at a high level. Almost every reaction is catalyzed by its own enzyme. What are enzymes? What is their role in the cell?
Enzymes. Definition
The term "enzyme" comes from the Latin fermentum - leaven. They can also be called enzymes from the Greek en zyme - “in yeast”.
Enzymes are biologically active substances, so any reaction occurring in a cell cannot occur without their participation. These substances act as catalysts. Accordingly, any enzyme has two main properties:
1) The enzyme accelerates the biochemical reaction, but is not consumed.
2) The value of the equilibrium constant does not change, but only accelerates the achievement of this value.
Enzymes speed up biochemical reactions a thousand, and in some cases a million, times. This means that in the absence of the enzymatic apparatus, all intracellular processes will practically stop, and the cell itself will die. Therefore, the role of enzymes as biologically active substances is great.
The variety of enzymes allows for versatile regulation of cell metabolism. Many enzymes of different classes take part in any reaction cascade. Biological catalysts are highly selective due to the specific conformation of the molecule. Since enzymes in most cases are protein in nature, they are in a tertiary or quaternary structure. This is again explained by the specificity of the molecule.
Functions of enzymes in the cell
The main task of the enzyme is to accelerate the corresponding reaction. Any cascade of processes, from the decomposition of hydrogen peroxide to glycolysis, requires the presence of a biological catalyst.
The correct functioning of enzymes is achieved by high specificity to a specific substrate. This means that a catalyst can only accelerate a certain reaction and no other, even very similar ones. According to the degree of specificity, the following groups of enzymes are distinguished:
1) Enzymes with absolute specificity, when only one single reaction is catalyzed. For example, collagenase breaks down collagen, and maltase breaks down maltose.
2) Enzymes with relative specificity. This includes substances that can catalyze a certain class of reactions, for example, hydrolytic cleavage.
The work of a biocatalyst begins from the moment its active center attaches to the substrate. In this case, they talk about complementary interaction like a lock and key. Here we mean the complete coincidence of the shape of the active center with the substrate, which makes it possible to accelerate the reaction.
The next stage is the reaction itself. Its speed increases due to the action of an enzymatic complex. Ultimately, we get an enzyme that is associated with the reaction products.
The final stage is the detachment of the reaction products from the enzyme, after which the active center again becomes free for the next job.
Schematically, the work of the enzyme at each stage can be written as follows:
1) S + E ——> SE
2) SE ——> SP
3) SP ——> S + P, where S is the substrate, E is the enzyme, and P is the product.

Classification of enzymes
A huge number of enzymes can be found in the human body. All knowledge about their functions and operation was systematized, and as a result, a single classification emerged, thanks to which you can easily determine what a particular catalyst is intended for. The 6 main classes of enzymes are presented here, as well as examples of some of the subgroups.
- Oxidoreductases.
Enzymes of this class catalyze redox reactions. A total of 17 subgroups are distinguished. Oxidoreductases usually have a non-protein part, represented by a vitamin or heme.
Among the oxidoreductases, the following subgroups are often found:
a) Dehydrogenases. The biochemistry of dehydrogenase enzymes involves the removal of hydrogen atoms and their transfer to another substrate. This subgroup is most often found in the reactions of respiration and photosynthesis. Dehydrogenases necessarily contain a coenzyme in the form of NAD/NADP or flavoproteins FAD/FMN. Metal ions are often found. Examples include enzymes such as cytochrome reductase, pyruvate dehydrogenase, isocitrate dehydrogenase, as well as many liver enzymes (lactate dehydrogenase, glutamate dehydrogenase, etc.).
b) Oxidases. A number of enzymes catalyze the addition of oxygen to hydrogen, as a result of which the reaction products can be water or hydrogen peroxide (H 2 0, H 2 0 2). Examples of enzymes: cytochrome oxidase, tyrosinase.
c) Peroxidases and catalases are enzymes that catalyze the decomposition of H 2 O 2 into oxygen and water.
d) Oxygenases. These biocatalysts accelerate the addition of oxygen to the substrate. Dopamine hydroxylase is one example of such enzymes.
2. Transferases.
The task of enzymes of this group is to transfer radicals from a donor substance to a recipient substance.
a) Methyltransferases. DNA methyltransferases are the main enzymes that control the process of nucleotide replication and play a large role in regulating the functioning of nucleic acids.
b) Acyltransferases. Enzymes of this subgroup transport an acyl group from one molecule to another. Examples of acyltransferases: lecithin cholesterol acyltransferase (transfers a functional group from a fatty acid to cholesterol), lysophosphatidylcholine acyltransferase (transfers an acyl group to lysophosphatidylcholine).
c) Aminotransferases are enzymes that are involved in the conversion of amino acids. Examples of enzymes: alanine aminotransferase, which catalyzes the synthesis of alanine from pyruvate and glutamate by amino group transfer.
d) Phosphotransferases. Enzymes of this subgroup catalyze the addition of a phosphate group. Another name for phosphotransferases, kinases, is much more common. Examples include enzymes such as hexokinases and aspartate kinases, which add phosphorus residues to hexoses (most often glucose) and aspartic acid, respectively.
3. Hydrolases - a class of enzymes that catalyze the cleavage of bonds in a molecule with the subsequent addition of water. Substances that belong to this group are the main digestive enzymes.
a) Esterases - break ether bonds. An example is lipases, which break down fats.
b) Glycosidases. The biochemistry of enzymes of this series consists in the destruction of glycosidic bonds of polymers (polysaccharides and oligosaccharides). Examples: amylase, sucrase, maltase.
c) Peptidases are enzymes that catalyze the breakdown of proteins into amino acids. Peptidases include enzymes such as pepsins, trypsin, chymotrypsin, and carboxypeptidase.
d) Amidases - cleave amide bonds. Examples: arginase, urease, glutaminase, etc. Many amidase enzymes are found in
4. Lyases are enzymes that are similar in function to hydrolases, but the cleavage of bonds in molecules does not require water. Enzymes of this class always contain a non-protein part, for example, in the form of vitamins B1 or B6.
a) Decarboxylase. These enzymes act on the C-C bond. Examples include glutamate decarboxylase or pyruvate decarboxylase.
b) Hydratases and dehydratases are enzymes that catalyze the reaction of cleavage of C-O bonds.
c) Amidine lyases - destroy C-N bonds. Example: arginine succinate lyase.
d) P-O lyase. Such enzymes, as a rule, cleave a phosphate group from a substrate substance. Example: adenylate cyclase.

The biochemistry of enzymes is based on their structure
The abilities of each enzyme are determined by its individual, unique structure. Any enzyme is first and foremost a protein, and its structure and degree of folding play a decisive role in determining its function.
Each biocatalyst is characterized by the presence of an active center, which, in turn, is divided into several independent functional areas:
1) The catalytic center is a special region of the protein through which the enzyme attaches to the substrate. Depending on the conformation of the protein molecule, the catalytic center can take on a variety of shapes, which must fit the substrate just like a lock fits a key. This complex structure explains what is in the tertiary or quaternary state.
2) Adsorption center - acts as a “holder”. Here, first of all, the connection between the enzyme molecule and the substrate molecule occurs. However, the bonds formed by the adsorption center are very weak, which means that the catalytic reaction at this stage is reversible.
3) Allosteric centers can be located both in the active center and over the entire surface of the enzyme as a whole. Their function is to regulate the functioning of the enzyme. Regulation occurs with the help of inhibitor molecules and activator molecules.

Activator proteins, by binding to the enzyme molecule, speed up its work. Inhibitors, on the other hand, inhibit catalytic activity, and this can happen in two ways: either the molecule binds to an allosteric site in the region of the active site of the enzyme (competitive inhibition), or it attaches to another region of the protein (non-competitive inhibition). considered more effective. After all, this closes the place for the substrate to bind to the enzyme, and this process is possible only in the case of an almost complete coincidence of the shape of the inhibitor molecule and the active center.
An enzyme often consists not only of amino acids, but also of other organic and inorganic substances. Accordingly, apoenzyme is the protein part, coenzyme is the organic part, and cofactor is the inorganic part. The coenzyme can be represented by carbohydrates, fats, nucleic acids, and vitamins. In turn, a cofactor is most often auxiliary metal ions. The activity of enzymes is determined by its structure: additional substances included in the composition change the catalytic properties. Various types of enzymes are the result of a combination of all the listed factors in the formation of the complex.

Regulation of enzymes
Enzymes as biologically active substances are not always necessary for the body. The biochemistry of enzymes is such that they can, if catalyzed excessively, harm a living cell. To prevent the harmful effects of enzymes on the body, it is necessary to somehow regulate their work.
Since enzymes are protein in nature, they are easily destroyed at high temperatures. The denaturation process is reversible, but it can significantly affect the performance of substances.
pH also plays a big role in regulation. The highest enzyme activity is usually observed at neutral pH values (7.0-7.2). There are also enzymes that work only in acidic environments or only in alkaline environments. Thus, a low pH is maintained in cellular lysosomes, at which the activity of hydrolytic enzymes is maximum. If they accidentally enter the cytoplasm, where the environment is already closer to neutral, their activity will decrease. This protection against “self-eating” is based on the peculiarities of the work of hydrolases.
It is worth mentioning the importance of coenzyme and cofactor in the composition of enzymes. The presence of vitamins or metal ions significantly affects the functioning of some specific enzymes.

Enzyme nomenclature
All enzymes in the body are usually named depending on their belonging to any of the classes, as well as on the substrate with which they react. Sometimes not one, but two substrates are used in the name.
Examples of the names of some enzymes:
- Liver enzymes: lactate dehydrogenase, glutamate dehydrogenase.
- Full systematic name of the enzyme: lactate-NAD+-oxidoreductase.
Trivial names that do not adhere to the rules of nomenclature have also been preserved. Examples are digestive enzymes: trypsin, chymotrypsin, pepsin.
Enzyme synthesis process
The functions of enzymes are determined at the genetic level. Since the molecule is, by and large, a protein, its synthesis exactly repeats the processes of transcription and translation.
Enzyme synthesis occurs according to the following scheme. First, information about the desired enzyme is read from DNA, resulting in the formation of mRNA. Messenger RNA encodes all the amino acids that make up the enzyme. Regulation of enzymes can also occur at the DNA level: if the product of the catalyzed reaction is sufficient, gene transcription stops and vice versa, if there is a need for the product, the transcription process is activated.
After the mRNA has entered the cytoplasm of the cell, the next stage begins - translation. On the ribosomes of the endoplasmic reticulum, the primary chain is synthesized, consisting of amino acids connected by peptide bonds. However, the protein molecule in the primary structure cannot yet perform its enzymatic functions.
The activity of enzymes depends on the structure of the protein. On the same EPS, protein twisting occurs, as a result of which first secondary and then tertiary structures are formed. The synthesis of some enzymes stops already at this stage, but to activate catalytic activity it is often necessary to add a coenzyme and a cofactor.
In certain areas of the endoplasmic reticulum, the organic components of the enzyme are added: monosaccharides, nucleic acids, fats, vitamins. Some enzymes cannot work without the presence of a coenzyme.
The cofactor plays a crucial role in the formation of Some enzyme functions are available only when the protein reaches a domain organization. Therefore, the presence of a quaternary structure, in which the connecting link between several protein globules is a metal ion, is very important for them.

Multiple Forms of Enzymes
There are situations when it is necessary to have several enzymes that catalyze the same reaction, but differ from each other in some parameters. For example, an enzyme can work at 20 degrees, but at 0 degrees it will no longer be able to perform its functions. What should a living organism do in such a situation at low ambient temperatures?
This problem is easily solved by the presence of several enzymes that catalyze the same reaction, but operate under different conditions. There are two types of multiple forms of enzymes:
- Isoenzymes. Such proteins are encoded by different genes, consist of different amino acids, but catalyze the same reaction.
- True plural forms. These proteins are transcribed from the same gene, but modification of the peptides occurs on the ribosomes. The output is several forms of the same enzyme.
As a result, the first type of multiple forms is formed at the genetic level, while the second type is formed at the post-translational level.
The importance of enzymes
In medicine, it comes down to the release of new medicines, which already contain substances in the required quantities. Scientists have not yet found a way to stimulate the synthesis of missing enzymes in the body, but today there are widespread drugs that can temporarily compensate for their deficiency.
Various enzymes in the cell catalyze a large number of reactions associated with maintaining life. One of these enisms are representatives of the group of nucleases: endonucleases and exonucleases. Their job is to maintain a constant level of nucleic acids in the cell and remove damaged DNA and RNA.
Don't forget about the phenomenon of blood clotting. As an effective protective measure, this process is controlled by a number of enzymes. The main one is thrombin, which converts the inactive fibrinogen protein into active fibrin. Its threads create a kind of network that clogs the site of vessel damage, thereby preventing excessive blood loss.
Enzymes are used in winemaking, brewing, and the production of many fermented milk products. Yeast can be used to produce alcohol from glucose, but an extract from it is sufficient for this process to proceed successfully.

Interesting facts you didn't know about
All enzymes in the body have a huge mass - from 5000 to 1,000,000 Da. This is due to the presence of protein in the molecule. For comparison: the molecular weight of glucose is 180 Da, and carbon dioxide is only 44 Da.
To date, more than 2000 enzymes have been discovered that have been found in the cells of various organisms. However, most of these substances have not yet been fully studied.
Enzyme activity is used to produce effective washing powders. Here, enzymes perform the same role as in the body: they break down organic matter, and this property helps in the fight against stains. It is recommended to use such washing powder at a temperature no higher than 50 degrees, otherwise denaturation may occur.
According to statistics, 20% of people around the world suffer from a deficiency of any of the enzymes.
The properties of enzymes were known for a very long time, but only in 1897 did people realize that not the yeast itself, but an extract from its cells, could be used to ferment sugar into alcohol.
Enzymes of microbial synthesis that break down proteins and carbohydrates
Amylorizin Sh1X and P10X. Dried cultural mass of the fungus Aspergillus avamori. Contains pectinesterase, dextrinase, glucoamylase, acid protease, cellulase, hemicellulase. Add to feed at the rate of 0.015% dry weight.
Pectavamorin PX, PZH, P10X (Avamorin P). Dried cultural mass of the fungus Aspergillus avamori. Contains pectinesterase, polymethylgalacteronase, acid protease, cellulase, hemicellulase. Add to feed at the rate of 0.015% dry weight.
Protosubtilin GZH. Bacterial protease. Added to cattle feed for better absorption of proteins, carbohydrates and fats at the rate of 0.01% dry weight.
Acid protease G10X. A proteolytic enzyme preparation obtained by deep cultivation of the fungus Aspergillus fostidus OV-208 by filtering the culture liquid, precipitating the filtrate with acetone and vacuum drying. IN
1 g contains 100 units. Added to calves feed at the rate of
0.2 g/feed units
When treating animals with infectious diseases, enzyme preparations are used that lyse components of the wall of microorganisms, which consists mainly of heteroisomo-peptidoglycan, of which 95% is contained in the wall of gram-positive microorganisms (staphylococci and streptococci), and only 10% in the wall of gram-negative bacteria. Lysis of the wall of microorganisms is carried out by three types of enzymes: amylase, glycosidases, proteases. Proteases, simultaneously with the lysis of protein components of the wall, activate intracellular bacterial enzymes that cause intracellular lysis, which enhances the antimicrobial effect.
In gynecological practice, lysozyme is used to treat patients with follicular vestibulitis, endometritis, and trichomoniasis. For infectious pathologies where staphylococci are the etiological factor, the enzyme preparation lysostatin is used, and for mycotic lesions of the birth canal (candidiasis, aspergillosis, etc.), copran and bolbit, obtained from lower fungi, are prescribed. Proteolytic enzymes of animal (trypsin, chymotrypsin, pepsin, etc.) and plant origin act for a short time in the treatment of purulent-necrotic processes. Since viable cells contain many anti-enzyme compounds, they are not subject to lysis.
Immobilized enzymes are more effective. Profenzyme is immobilized on water-soluble cellulose granules, and the complex proteolytic drug imosin is immobilized on a water-soluble polymer.
Lysosubtilin G10X (Lysosubtilinum G10X). Light yellow fine powder, highly soluble in water. Its lytic activity is measured in units (1 million units in 1 g). The powder is produced in glass tubes of 10 g and in bags of 500, 1000 and 2000 g.
Used in the treatment of endometritis in cows. For this
2 million units are dissolved in 100 ml of distilled water and injected into
uterus To prevent diarrhea in calves, the drug is added to
colostrum (milk) 20 g/l 2 times a day for 8-10 days. For medicinal purposes, add 25 g to milk 2 times a day until recovery.
Lysozyme GZH (Lysocimum). Light gray odorless powder, soluble in water. An enzyme preparation obtained by drying the culture liquid of lysozyme producers. Contains proteolytic enzymes.
They produce powder packaged in 15 kg bags. Entered inside. In gram-positive and gram-negative microorganisms, it lyses the wall built of polyaminosaccharides. Improves the breakdown and resorption of nutrients. Strengthens the phagocytic activity of neutrophils and stimulates proliferative processes.
Used for fattening broiler chickens, adding to feed at the rate of 0.3% dry weight.
Calves are prescribed in complex therapy for bronchopneumonia, diarrhea, osteodystrophy at a dose of 0.15-0.2 g/kg animal weight for 5-20 days. When treating calves with dystrophy, the drug is added to milk at a dose of 2-4 g/l 3 times a day.
Pepsinorm. Enzyme-bacterial preparation.
They are produced in two forms: pepsinorm-1, a grayish-yellow liquid with a specific odor, and pepsinorm-2, a cream-colored powdery mass, partially soluble in water to form homogeneous suspensions.
Normalizes digestion and eliminates dysbiosis due to the presence of bacteriolytic and proteolytic enzymes.
Used for the treatment and prevention of acute gastrointestinal diseases in newborn calves. For medicinal purposes 50- " 100 ml of liquid or 0.5-1 g of dry pepsinorm is mixed with 0.5 liters of colostrum (milk) and heated. At the same time, specific therapeutic agents can be prescribed.