A month and a half ago, we wrote about the fact that...
Where did the radioactive cloud come from in Europe? At the same time, it was suggested that the source was in Eastern Europe, in particular in Russia, between the Volga and the Urals.
And here’s what’s interesting: the Western media, the public, scientists are sounding the alarm, but we know nothing. This is weird. However, it’s common. We didn’t know anything about Chernobyl for several days. And only today, when we were finally backed, as they say, against the wall, we started talking about it. The entire federal agenda is full of ruthenium-106.
Information appeared in the media that in some settlements (for example, in the Tsimlyansky region) the permissible concentration of this isotope exceeded the norm by 139 times, in Morozovsk - by 27 times. As for Rostov, it is still unknown. Although if the cloud flew and reached France and the Mediterranean countries, then it certainly did not pass us by.
It’s a shame that we are again being taken, as they say, for suckers. Although imagine: they would have reported immediately - what a panic would have arisen. Otherwise, they could just quietly die from an unknown cause if something happened.
Another amazing thing is this. The location of distribution is known - this is, with a high probability, the notorious Mayak enterprise in the Chelyabinsk region. But Rosatom denies: there were no accidents. Someone specially brought an isotope and sprayed it near Mayak, and in such quantities that it reached Europe?!
However, let's leave these rhetorical questions aside. You need to think - what to do, if something can be done. So, what is ruthenium and should we be afraid of it?
Ruthenium 106 is a radioactive substance used in radiation therapy for tumors, as well as in satellite power generators and small nuclear reactors.
Naturally, at a certain level of background radiation it becomes dangerous. It acts on the human body in the same way as cesium-137. And, by the way, the consequences of its influence do not appear immediately, but after about six months.
The Great Medical Encyclopedia reports that the critical organ for soluble compounds of radioactive isotopes of R. is the gastrointestinal tract, and for insoluble compounds - in some cases, the gastrointestinal tract and lungs.
This is a radioactive isotope of ruthenium with a half-life of just over a year (373.6 days), subject to beta decay to rhodium-106, which then almost immediately undergoes another beta decay to stable palladium-106. These two beta decays produce electrons of a wide range of energies up to about 3 MeV and are accompanied by gamma radiation (the strongest lines are 511 and 622 keV). Accordingly, it is dangerous due to its radioactivity and radiotoxicity. And protection is the same as from any other radioactive isotopes: from external radiation - time, distance and, if necessary, shielding with lead, from internal radiation - taking all possible measures against its entry into the body by strictly observing all the rules for working with open sources of ionizing radiation.
As for the recent ruthenium-106 release, scientists say there is no need to worry. Its concentration where it was discovered is negligible and many times lower than those concentrations of radioactive isotopes (primarily radon and its decay products) that we constantly encounter, so there is no need to protect ourselves from it. It’s just hard to believe in this.
Where, then, do these frightening figures about the excess of this radioactive isotope in the atmosphere by hundreds, and within the territory of contamination by almost a thousand times?
Meanwhile, Interfax reports continued to arrive throughout the day: the recorded release of ruthenium-106 in the air does not exceed the maximum permissible concentrations, Roshydromet head Maxim Yakovenko told reporters.
“It was a fact, but the concentrations that were caught were hundreds to thousands of times lower than the maximum permissible concentrations. That is, there is no danger,” Yakovenko said.
“As for the source, we are not looking for the source for one simple reason: why look for it when there is no danger,” he added.
At the same time, the administration of the Chelyabinsk region reported that there was no panic in the region. And the Chelyabinsk Regional Oncology Center does not have information about dangerous concentrations of radiation in the region.
The region's chief oncologist, Andrei Vazhenin, told Interfax today.
“We are carrying out our monitoring. We have not received any information about the dangerous situation in the region,” he said. According to the oncologist, ruthenium-106 “is not a pure carcinogen.” “The population has nothing to worry about,” he emphasized.
Meanwhile, by the evening, rather alarming information began to leak out. Thus, RIA Novosti, citing Roshydromet, reported that at the end of September, extremely high air pollution with this radioactive isotope was observed in the Southern Urals, high pollution in Tatarstan, the Volga region and Rostov-on-Don.
But whether it is dangerous or not, whether someone was seriously exposed to radiation or not, we are unlikely to know from official sources. And the worst thing is that we can’t do anything here. Moreover, each of us has already received ours if the level of radiation pollution was really high. We have nowhere to go, to run away, to sail away, because the Earth is small, we cannot feel safe anywhere. However, there must be some kind of official message from the authorities or not?
And most importantly: we need to tell people what preventive measures are needed to protect themselves from possible consequences, and do this immediately at the state level.
I personally think that what, as they say, the Rostov region “grabbed” may indeed be harmless. But we also have a facility of increased radioactive danger - a nuclear power plant in Volgodonsk. God forbid, of course, but suddenly something happens - I’m almost sure that warning people and preventive measures against the possible consequences of radiation damage will be implemented as a last resort.
In recent months, Europe and Russia have been rocked by reports of a looming radioactive cloud of ruthenium-106. People wonder: what’s the matter, what happened?
The usual story. When something related to radioactivity happens, specialists working specifically in this field remain silent, and comments are made by people who have heard something about radioactive isotopes, but do not really understand.
At one time I had to work with radioactive isotopes of ruthenium and study their volatility. In general, the matter is clear.
1. How ruthenium-106 is obtained?
This radionuclide (half-life 374 days) is a fission product of uranium and is produced during the operation of nuclear reactors. They don’t get it at all at cyclotrons; talking about it is stupid.
The yield of ruthenium-106 in fission products is 0.4%, and another shorter-lived radioisotope of ruthenium, ruthenium-103 (half-life 39 days) is 3%. The chemical behavior of both radionuclides is the same, and if the second isotope is not visible (as in this case), this means that ruthenium-106 was released from the old products of the nuclear reactor, a year and a half or even several years after production.
2. How could a release of pure ruthenium-106 occur?
Pure ruthenium-106 is obtained in small quantities to make applicators for the treatment of certain eye diseases. But the appearance of a huge ruthenium cloud cannot be explained by some kind of processing of these medical products. The release was estimated to be 100-300 terabecquerels. This is a huge activity, no amount of applicators will be enough. And why recycle them?
Another “canard”: ruthenium appeared as a result of the destruction of a satellite. This is refuted by A.B., a member of the Russian Academy of Cosmonautics, a former adviser to the head of RSC Energia. Zheleznyakov: ruthenium-106 is not used on satellites.
So what's the matter? Why are no other uranium fission products visible?
The fact is that ruthenium has a chemical property that is quite rare for metals - it forms a highly volatile compound - ruthenium tetroxide. So when nuclear waste is heated in air to a certain temperature, only ruthenium will fly. There are other highly volatile fission products of uranium, such as iodine-131, but it has already decayed (half-life 8 days); Another isotope of iodine, iodine-129, has a very long half-life (16 million years), so its activity is extremely low and is not visible against this background.
Thus, if an aqueous solution of old radioactive waste is evaporated in air or heated in a vitrification furnace, only ruthenium-106 in the form of tetroxide will fly. Such long-lived radionuclides as strontium-90 and cesium-137 are not volatile under these conditions and therefore are not released when heated. They appear in the air either during an explosion and the release of a solid or liquid substance, or when heated to a much higher temperature - during the operation of a nuclear reactor. Existing technologies for processing radioactive waste, of course, provide for the capture of escaped ruthenium with special filters, but, apparently, in this case the filters did not work.
3. How ruthenium-106 spreads?
Once in the atmosphere, ruthenium will be deposited on dust particles in the form of low-volatile dioxide. The spread can be quite wide and the cloud can travel far depending on weather conditions. Partial precipitation of particles leads to an increased concentration of the radioisotope on the surface at certain points. Naturally, more such points will be close to the place where the release occurred, but ruthenium fallout can occur quite far from the accident site. Ruthenium-106 itself emits only beta particles, but its distribution can be easily traced by the gamma activity of its short-lived fission product, rhodium-106.
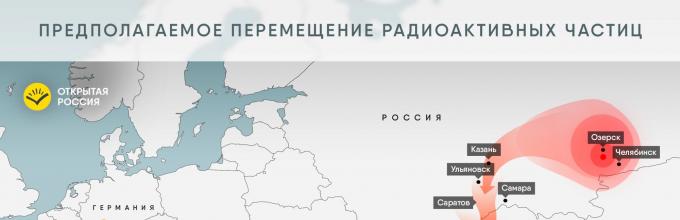
Figure 1. Initial distribution of ruthenium-106 activity according to calculations by the French Institute for Nuclear and Radiation Safety

Rice. 2. Movement of radioactive particles inferred from published measurement data4. Where could this happen??
The published maps show (see Fig. 1 and 2) that the cloud began its spread from the Ural region. Of the large nuclear facilities, the Mayak production association, an enterprise of the Rosatom state corporation in the city of Ozersk (Chelyabinsk region), is located there. Not so far away, near Yekaterinburg, there is the Beloyarsk Nuclear Power Plant, also a Rosatom enterprise. Most commentators suspect Mayak of the incident because it is there that they reprocess nuclear waste.
The points with the greatest contamination with ruthenium-106, according to the published bulletin of the Federal Service for Hydrometeorology and Environmental Monitoring of Russia (Roshydromet), are the villages of Metlino, Argayash, Khudayberdinsk, Novogorny - located precisely in these places, in the Chelyabinsk region. Mayak denies involvement in the accident and emissions. This enterprise is closed, unauthorized access to any of its facilities is strictly prohibited, so it is quite difficult to check them.
5. How dangerous is this for the population??
Authorities and experts say the detected concentrations of ruthenium-106 are not dangerous. Many people, remembering the Chernobyl story, do not believe them. Let's take a closer look.
Journalists and some environmentalists like to compare the level of pollution with the background value (as they say - the usual value). This is completely illegal. If the background value of a rare substance is close to zero, then a thousand times the background value means little.
It's not a matter of the presence of radioactivity, but the level of radioactivity. It is completely wrong to think that any radioactivity is harmful. Radioactivity is everywhere and always. At low doses, the number of diseases is not at all proportional to the radiation dose, rather the opposite (radiation hormesis). The human body needs this kind of immunity, otherwise it may die, for example, after solar flares.
There are standards [Radiation Safety Standards, NRB-99/2009 and SanPiN 2.6.1.2523-09, Moscow, 2009], they are quite strict and made with a large margin. According to these standards, for professionals working with radioactivity and under constant supervision (persons of category A), the maximum annual intake of ruthenium-103 into the body is up to 1,100,000 becquerels, in the workplace in the air it can be no more than 440 becquerels per cubic meter .
For category B persons - the entire population - the standards are more stringent - no more than 36,000 becquerels ingested and 4.4 becquerels per cubic meter on average per year. The radiotoxicity of ruthenium-106 is higher than that of cesium-137, but lower than that of strontium-90.
Ruthenium is an isotope, a chemical substance of natural or chemical origin. There are its varieties: natural (a stable substance belonging to the light metals of the platinum group), ruthenium-106 (an unstable radioactive substance formed as a side effect of the combustion of nuclear fuel). It is important to know the symptoms of ruthenium poisoning, methods of its treatment and prevention in order to avoid complications.
Ruthenium-106 is a radionuclide of artificial origin, which arises during the decay of uranium during the release of energy in nuclear power plants. The element has a nuclear half-life of 1 year, which classifies it as a dangerous radioactive isotope. You can be exposed to ruthenium-106 against a background of increased background radiation; in medicine it is used to treat oncology, but if poisoned, it becomes the cause of malignant tumors. Soluble isotope compounds are poisoned through the gastrointestinal tract, insoluble compounds are poisoned through the gastrointestinal tract and lungs.
The degree of ruthenium poisoning depends on many factors: radiation dose, the duration of a person’s stay at the site of the accident or chemical emissions, and the time of work in platinum group deposits. The effect of the substance on the body is local in nature, affecting the organ that was in direct contact with the poison.
Ruthenium (Ru) is a metal that can be poisoned by inhaling its salts through direct physical contact or ingesting them. Both substances are extremely rare in nature; they are mainly synthesized in laboratory conditions for ophthalmic and radiation medical and industrial purposes.
Professionals who come into contact with ruthenium salts have the following occupational diseases:
- eczema of the hands;
- contact dermatitis;
- hives;
- conjunctivitis;
- allergic blepharitis;
- respiratory diseases.
People affected by ruthenium-106 suffer from general intoxication of the body, which increases the likelihood of tumor diseases, deterioration in a person’s physical condition, hair loss, and genetic mutations.

Symptoms and signs of poisoning
The toxic effect of ruthenium in the form of specific ulcers in the nasopharynx and mucous membranes of the respiratory tract is observed in most people working with metal salts. The first signs of ruthenium poisoning are: cough and sore throat. Unlike poisoning with other chemicals, victims of platinoid do not have prodromal symptoms - sneezing, nosebleeds, swelling and burning of the nasal mucosa. Symptoms of poisoning are the body’s reactions to nitrates formed on human mucous membranes: irritation of the eyes, respiratory tract, skin dermatoses.
If platinum salts enter the body through the digestive system, then within the first 2 days up to 95% of the substance will be excreted in the feces, the rest is excreted within one to two months. Platinoid salts tend to circulate in the blood for a long time with low absorption from the gastrointestinal tract. Absorption ranges from 1 to 13%. The urinary system is practically not involved when ridding the body of ruthenium salts.
In case of ruthenium-106 poisoning, symptoms appear after six months and depend on the severity of intoxication. Ru-106 increases the risk of cancer or accelerates its development. Symptoms appear individually, depending on the affected organ. Minor exposure may result in temporary fatigue, weakness, hair loss, or allergic skin or respiratory reactions. When aerosols are inhaled, the respiratory organs bear the brunt of the impact - 50% of harmful substances settle in the lungs.
According to Russian sanitary standards, SanPiN 2.6.1.2523-09 on radioactive safety of the population, the maximum concentration of the isotope entering the human body is limited:
At the end of 2017, they started talking about the discovery of ruthenium-106 in air masses. At that concentration, poisoning can be obtained by inhaling 1 million cubic meters of contaminated air for an ordinary person (for a professional - 100 million cubic meters), despite the fact that a person inhales only a few thousand cubic meters per year.

In the professional sphere, when working with chemicals, the concept of “ruthenium 106 poisoning” constitutes a significant part of the safety and labor protection instructions. At the first signs of poisoning, in order to avoid complications, the employee is removed from work; with minor damage, the symptoms quickly disappear; with severe intoxication, the symptoms worsen with each new contact. The pattern of exacerbations is cyclical and depends on the method of contact with the chemical.
Ruthenium antidote is a set of measures to prevent the substance from entering or entering the human respiratory tract. It is recommended to wash your hands before eating, smoking, and after working with chemicals. In case of accidents at large enterprises where ruthenium synthesis is carried out, the population is evacuated and screens made of lead are constructed to protect the population from radiation exposure. First aid consists of evacuating the victim from the site of poisoning and alleviating symptoms. In case of intoxication, doctors prescribe symptomatic treatment, since there is no antidote or antidote as a specific drug therapy.
A certain level of radiation is always present in varying concentrations in all corners of the Earth, which is the norm. A temporary increase in background radiation to the maximum permissible norm does not cause any deterioration in the health of the population - humanity is exposed to solar radiation every day. Its peculiarity is different: they are afraid of radiation, and the fear of an invisible enemy causes panic in a person.
Since the radioactive cloud settles unevenly, it is important for scientists during an environmental disaster to monitor the level of contamination, taking the necessary measures to evacuate the population.
Consequences of radioactive metal poisoning
The consequences of ruthenium poisoning in hazardous industries are minimized. Preventive measures, modern equipment and automation of production processes reduce the likelihood of mass poisonings. If physical contact with ruthenium salts is necessary, specialists use special protective clothing and respirators, the removal of which from the territory of the enterprise is not allowed. Poisoning prevention measures include regular medical examinations and monitoring the condition of victims. To prevent ruthenium from getting on the skin of the hands, special creams are used, and a shower is mandatory at the end of the working day.
If radioactive substances are found in the air of populated areas due to leakage or emissions into the atmosphere, the contamination factor is higher the longer the duration of the person at the site of contamination. When ruthenium is ingested through the gastrointestinal tract, pectins contained in fresh fruits and vegetables are indicated: apples, pears, kiwis, mangoes, bananas, plums, figs, strawberries, blueberries, carrots. There are a number of medications that absorb and remove dangerous chemicals from the body.
In ordinary life, the likelihood of getting ruthenium poisoning is low. If an accident occurs at a nuclear power plant, we will talk about irradiation with other, more dangerous isotopes.
They do not exist in nature, but they are formed as a result of the fission of uranium and plutonium nuclei in reactors of nuclear power plants, submarines, ships, and during explosions of atomic bombs. Most radioactive isotopes of ruthenium are short-lived, but two - ruthenium-103 and ruthenium-106 - have fairly long half-lives (39.8 days and 1.01 years) and accumulate in reactors. It is significant that during the decay of plutonium, ruthenium constitutes up to 30% of the total mass of all fission fragments. From a theoretical point of view, this fact is certainly interesting. It even has a special “zest”: the alchemists’ dream came true - base metal turned into noble one. Indeed, these days, plutonium production plants throw out tens of kilograms of the noble metal ruthenium. But the practical harm caused by this process to nuclear technology would not pay off even if it were possible to put to good use all the energy produced in nuclear reactors.
Why is ruthenium harmful?
One of the main advantages of nuclear fuel is its reproducibility. As is known, when uranium blocks are “burned” in nuclear reactors, new nuclear fuel is formed - . At the same time, “ash” is also formed - fragments of the fission of uranium nuclei, including ruthenium. The ash, of course, has to be removed. Not only do the nuclei of fragmentation elements capture neutrons and break the chain reaction, they also create radiation levels that significantly exceed permissible limits. It is relatively easy to separate the bulk of fragments from uranium and plutonium, which is done in special plants, but radioactive ones cause a lot of trouble.
Unspent and fragments are separated in special installations. The first stage of separation is the dissolution of uranium blocks in nitric acid. This is where the troubles with ruthenium begin. When dissolved, part of it turns into complex nitroso compounds, which are based on the trivalent group (RuNO)3+. This group forms complex compounds of various compositions in nitric acid. They interact with each other or with other ions in solution, hydrolyze, or even combine into inorganic polymer molecules. The complexes are completely different, but it is very difficult to separate and identify them. The endless variety of properties of ruthenium nitroso compounds poses many difficult questions to chemists and technologists.
There are several methods for separating fragments from plutonium and uranium. One of them is ion exchange. A solution containing various ions passes through a system of ion exchangers. The meaning of this operation is that they are retained by ion exchangers in the apparatus, and other elements pass freely through the entire system. However, it only partially goes away. Some of it remains on the ion exchanger along with the uranium.
In another method - precipitation - it is transferred to a precipitate with special reagents, and the fragments remain in solution. But along with uranium, part of the ruthenium also precipitates.
When purified by extraction, uranium is extracted from an aqueous solution with organic solvents, for example esters of organophosphorus acids. The fragments remain in the aqueous phase, but not all of them - ruthenium partially passes into the organic phase along with uranium.
They tried to avoid the difficulties of purifying nuclear fuel from ruthenium by using dry methods that eliminated the dissolution of uranium blocks. Instead of nitric acid, they were treated with fluorine. It was assumed that the uranium would then transform into volatile hexafluoride and be separated from the non-volatile fluorides of fragmentation elements. But ruthenium remained true to itself here too. It turned out that it also forms volatile fluorides.
Difficulties with ruthenium haunt technologists at the next stages of working with fissile materials. When collecting fragments from waste solutions, most of the foreign elements can be transferred to sediment, and ruthenium again partially remains in solution. Biological treatment does not guarantee its removal when waste solutions are poured into special drainless reservoirs.
Ruthenium begins to gradually migrate into the ground, creating the danger of radioactive contamination at large distances from the reservoir. the same thing happens when fragments are buried in mines at great depths. Radioactive ruthenium, which has (in the form of water-soluble nitroso compounds) extreme mobility, or, more correctly, migration ability, can travel very far with groundwater.
The problem of cleaning - decontamination of equipment, clothing, etc. - from radioruthenium also has its own specifics. Depending on the chemical state in which ruthenium was, it can either be easily washed and removed, or it can be deactivated with great difficulty.
Physicists, chemists, technologists, and especially radiochemists in many countries pay a lot of attention to the fight against radioactive ruthenium. At the I and II International Conferences on the Peaceful Uses of Atomic Energy in Geneva, several reports were devoted to this problem. However, there is still no reason to consider the fight against ruthenium completed successfully, and, apparently, chemists will have to work a lot more in order for this problem to be transferred to the category of finally solved.
Where does the ruthenium-106 isotope come from and how dangerous is it? Doctor of Chemical Sciences, Professor V.G. told euronews about this. Trades from .
Ruthenium-106 (Ru-106) is a radionuclide (i.e., an atom with an unstable nucleus prone to spontaneous transformation (radioactive decay), accompanied by ionizing radiation) of artificial origin. “Ru-106 is a product that is obtained during the operation of nuclear power plants. When energy is released, uranium disintegrates and fragmented metals are obtained. Ruthenium is a decay product of uranium-235, roughly speaking,” says the scientist.
Professor V.G. Torgov calls Ruthenium-106 a “long-lived isotope” because it has a long decay period. “A radioactive atom decays slowly,” notes Professor V.G. Torg, “It is believed that the longer the half-life, the more dangerous the radioactive isotope.” The half-life of Ru-106 is one year.
“But still, the main danger of Ruthenium-106 is radiation exposure, just like from any other radioactive isotope,” the scientist continues. That is, exposure to ruthenium can increase the risk of developing cancer or radiation sickness.” “Ruthenium, cesium, cobalt are the most dangerous radioactive isotopes, since they are more durable,” says the researcher.
Ru-106 compounds are volatile, “so when it goes into the atmosphere, it doesn’t end up anywhere,” says the professor.
Ru-106” was somehow allocated in advance for some purpose”
Ru-106, as a by-product of the decay of uranium-235, is isolated from the environment using special technology. It's called vitrification. Warp method- conversion of radioactive waste into a glass-like form at high temperatures.
“The vitrification furnace contains measures to prevent the volatility of all volatile compounds. A reducing agent is added there, which also converts ruthenium oxide into an absolutely non-volatile metal. Therefore, in principle, there can be no ruthenium emissions from this stove simply due to technology. Everything there is done so that nothing happens,” says the professor in an interview with euronews.
“The uniqueness of the situation here, first of all, is that an excess of only one radionuclide was recorded - ruthenium-106,” notes I. Smirnov. “This event cannot in any way be connected either with nuclear energy or with the reprocessing of irradiated nuclear fuel, because there is no no other components. In the event of some kind of accident at a station or at a production facility during fuel processing, all the radionuclides that were formed fly out.”
In the case of the release of ruthenium-106, according to Professor I. Smirnov, “this means that it was somehow allocated in advance for some purpose.”
History of ruthenium
Ruthenium is the eighth element of the fifth period of the Mendleyev periodic table. This noble metal became known to the world in the middle of the 19th century, when it was discovered by Kazan University professor Karl Klaus. The scientist isolated ruthenium from Ural platinum ore in its pure form and named the new element in honor of Russia (lat. Ruthenia).