Carbon dioxide (carbon dioxide), also called carbon dioxide, is the most important component in carbonated drinks. It determines the taste and biological stability of drinks, gives them sparkling and refreshing properties.
Chemical properties. Chemically, carbon dioxide is inert. Formed with the release of a large amount of heat, it, as a product of complete oxidation of carbon, is very stable. Carbon dioxide reduction reactions occur only at high temperatures. So, for example, interacting with potassium at 230° C, carbon dioxide is reduced to oxalic acid:
Entering into a chemical interaction with water, the gas, in an amount of no more than 1% of its content in the solution, forms carbonic acid, which dissociates into H +, HCO 3 -, CO 2 3- ions. In an aqueous solution, carbon dioxide easily enters into chemical reactions, forming various carbon dioxide salts. Therefore, an aqueous solution of carbon dioxide is highly aggressive towards metals and also has a destructive effect on concrete.
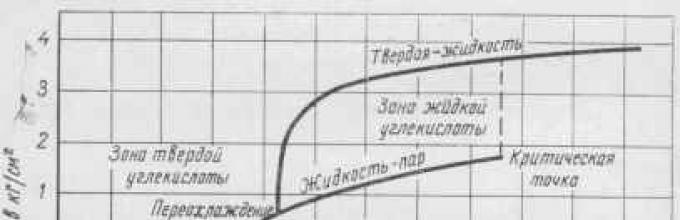
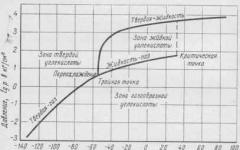
Physical properties. To carbonate drinks, carbon dioxide is used, brought to a liquid state by compression to high pressure. Depending on the temperature and pressure, carbon dioxide can also be in a gaseous or solid state. The temperature and pressure corresponding to this state of aggregation are shown in the phase equilibrium diagram (Fig. 13).

At a temperature of minus 56.6 ° C and a pressure of 0.52 Mn/m 2 (5.28 kg/cm 2), corresponding to the triple point, carbon dioxide can simultaneously be in a gaseous, liquid and solid state. At higher temperatures and pressures, carbon dioxide is in liquid and gaseous states; at temperatures and pressures that are below these values, the gas, directly bypassing the liquid phase, passes into the gaseous state (sublimates). At temperatures above the critical temperature of 31.5° C, no amount of pressure can keep carbon dioxide in liquid form.
In the gaseous state, carbon dioxide is colorless, odorless and has a mild sour taste. At a temperature of 0° C and atmospheric pressure, the density of carbon dioxide is 1.9769 kg/f 3 ; it is 1.529 times heavier than air. At 0°C and atmospheric pressure, 1 kg of gas occupies a volume of 506 liters. The relationship between volume, temperature and pressure of carbon dioxide is expressed by the equation:
where V is the volume of 1 kg of gas in m 3 /kg; T - gas temperature in ° K; P - gas pressure in N/m 2; R - gas constant; A is an additional value that takes into account the deviation from the equation of state of an ideal gas;

Liquefied carbon dioxide- a colorless, transparent, easily mobile liquid, reminiscent in appearance of alcohol or ether. The density of the liquid at 0°C is 0.947. At a temperature of 20°C, the liquefied gas is stored under a pressure of 6.37 Mn/m2 (65 kg/cm2) in steel cylinders. When the liquid flows freely from the cylinder, it evaporates, absorbing a large amount of heat. When the temperature drops to minus 78.5° C, part of the liquid freezes, turning into so-called dry ice. Dry ice is close to chalk in hardness and has a matte white color. Dry ice evaporates slower than liquid, and it immediately turns into a gaseous state.
At a temperature of minus 78.9 ° C and a pressure of 1 kg/cm 2 (9.8 MN/m 2), the heat of sublimation of dry ice is 136.89 kcal/kg (573.57 kJ/kg).
The most common processes for the formation of this compound are the rotting of animal and plant remains, the combustion of various types of fuel, and the respiration of animals and plants. For example, one person emits about a kilogram of carbon dioxide into the atmosphere per day. Carbon monoxide and dioxide can also be formed in inanimate nature. Carbon dioxide is released during volcanic activity and can also be produced from mineral water sources. Carbon dioxide is found in small quantities in the Earth's atmosphere.
The peculiarities of the chemical structure of this compound allow it to participate in many chemical reactions, the basis for which is carbon dioxide.
Formula
In the compound of this substance, the tetravalent carbon atom forms a linear bond with two oxygen molecules. The appearance of such a molecule can be represented as follows:

The hybridization theory explains the structure of the carbon dioxide molecule as follows: the two existing sigma bonds are formed between the sp orbitals of the carbon atoms and the two 2p orbitals of oxygen; The p-orbitals of carbon, which do not take part in hybridization, are bonded in conjunction with similar orbitals of oxygen. In chemical reactions, carbon dioxide is written as: CO 2.
Physical properties
Under normal conditions, carbon dioxide is a colorless, odorless gas. It is heavier than air, which is why carbon dioxide can behave like a liquid. For example, it can be poured from one container to another. This substance is slightly soluble in water - about 0.88 liters of CO 2 dissolve in one liter of water at 20 ⁰C. A slight decrease in temperature radically changes the situation - 1.7 liters of CO 2 can dissolve in the same liter of water at 17⁰C. With strong cooling, this substance precipitates in the form of snow flakes - the so-called “dry ice” is formed. This name comes from the fact that at normal pressure the substance, bypassing the liquid phase, immediately turns into a gas. Liquid carbon dioxide is formed at a pressure just above 0.6 MPa and at room temperature.

Chemical properties
When interacting with strong oxidizing agents, 4-carbon dioxide exhibits oxidizing properties. The typical reaction of this interaction is:
C + CO 2 = 2CO.
Thus, with the help of coal, carbon dioxide is reduced to its divalent modification - carbon monoxide.
Under normal conditions, carbon dioxide is inert. But some active metals can burn in it, removing oxygen from the compound and releasing carbon gas. A typical reaction is the combustion of magnesium:
2Mg + CO 2 = 2MgO + C.
During the reaction, magnesium oxide and free carbon are formed.

In chemical compounds, CO 2 often exhibits the properties of a typical acid oxide. For example, it reacts with bases and basic oxides. The result of the reaction is carbonic acid salts.
For example, the reaction of a compound of sodium oxide with carbon dioxide can be represented as follows:
Na 2 O + CO 2 = Na 2 CO 3;
2NaOH + CO 2 = Na 2 CO 3 + H 2 O;
NaOH + CO 2 = NaHCO 3.
Carbonic acid and CO 2 solution
Carbon dioxide in water forms a solution with a small degree of dissociation. This solution of carbon dioxide is called carbonic acid. It is colorless, weakly expressed and has a sour taste.
Recording a chemical reaction:
CO 2 + H 2 O ↔ H 2 CO 3.
The equilibrium is shifted quite strongly to the left - only about 1% of the initial carbon dioxide is converted into carbonic acid. The higher the temperature, the fewer carbonic acid molecules in the solution. When the compound boils, it disappears completely, and the solution disintegrates into carbon dioxide and water. The structural formula of carbonic acid is presented below.

Properties of carbonic acid
Carbonic acid is very weak. In solutions, it breaks down into hydrogen ions H + and compounds HCO 3 -. CO 3 - ions are formed in very small quantities.
Carbonic acid is dibasic, so the salts formed by it can be medium and acidic. In the Russian chemical tradition, medium salts are called carbonates, and strong salts are called bicarbonates.
Qualitative reaction
One possible way to detect carbon dioxide gas is to change the clarity of the lime mortar.
Ca(OH) 2 + CO 2 = CaCO 3 ↓ + H 2 O.
This experience is known from a school chemistry course. At the beginning of the reaction, a small amount of white precipitate is formed, which subsequently disappears when carbon dioxide is passed through water. The change in transparency occurs because during the interaction process, an insoluble compound - calcium carbonate - is converted into a soluble substance - calcium bicarbonate. The reaction proceeds along this path:
CaCO 3 + H 2 O + CO 2 = Ca(HCO 3) 2.
Production of carbon dioxide
If you need to get a small amount of CO2, you can start the reaction of hydrochloric acid with calcium carbonate (marble). The chemical notation for this interaction looks like this:
CaCO 3 + HCl = CaCl 2 + H 2 O + CO 2.
Also for this purpose, combustion reactions of carbon-containing substances, for example acetylene, are used:
CH 4 + 2O 2 → 2H 2 O + CO 2 -.
A Kipp apparatus is used to collect and store the resulting gaseous substance.
For the needs of industry and agriculture, the scale of carbon dioxide production must be large. A popular method for this large-scale reaction is to burn limestone, which produces carbon dioxide. The reaction formula is given below:
CaCO 3 = CaO + CO 2.
Applications of carbon dioxide
The food industry, after large-scale production of “dry ice,” switched to a fundamentally new method of storing food. It is indispensable in the production of carbonated drinks and mineral water. The CO 2 content in drinks gives them freshness and significantly increases their shelf life. And carbidization of mineral waters allows you to avoid mustiness and unpleasant taste.

In cooking, the method of extinguishing citric acid with vinegar is often used. The carbon dioxide released gives fluffiness and lightness to confectionery products.
This compound is often used as a food additive to increase the shelf life of food products. According to international standards for the classification of chemical additives contained in products, it is coded E 290,
Powdered carbon dioxide is one of the most popular substances included in fire extinguishing mixtures. This substance is also found in fire extinguisher foam.
It is best to transport and store carbon dioxide in metal cylinders. At temperatures above 31⁰C, the pressure in the cylinder can reach critical and liquid CO 2 will go into a supercritical state with a sharp rise in operating pressure to 7.35 MPa. The metal cylinder can withstand internal pressure up to 22 MPa, so the pressure range at temperatures above thirty degrees is considered safe.
A qualitative reaction for detecting carbon dioxide is the turbidity of lime water:
Ca(OH)2 + CO2 = CaCO3↓ + H2O.
At the beginning of the reaction, a white precipitate is formed, which disappears when CO2 is passed through lime water for a long time, because insoluble calcium carbonate turns into soluble bicarbonate:
CaCO3 + H2O + CO2 = Ca(HCO3)2.
Receipt. Carbon dioxide is obtained by thermal decomposition of carbonic acid salts (carbonates), for example, by burning limestone:
CaCO3 = CaO + CO2,
or by the action of strong acids on carbonates and bicarbonates:
CaCO3 + 2HCl = CaCl2 + H2O + CO2,
NaHCO3 + HCl = NaCl + H2O + CO2.
Carbon emissions, sulfur compounds into the atmosphere as a result of industrial activity, the functioning of energy and metallurgical enterprises lead to the occurrence of the greenhouse effect and associated climate warming.
Scientists estimate that global warming without measures to reduce greenhouse gas emissions will range from 2 to 5 degrees over the next century, which will be an unprecedented phenomenon in the last ten thousand years. Climate warming and an increase in sea level by 60-80 cm by the end of the next century will lead to an environmental disaster of unprecedented scale, which threatens the degradation of the human community.
Carbonic acid and its salts. Carbonic acid is very weak, exists only in aqueous solutions and slightly dissociates into ions. Therefore, aqueous solutions of CO2 have slightly acidic properties. Structural formula of carbonic acid:
As a dibasic, it dissociates stepwise: H2CO3H++HCO-3 HCO-3H++CO2-3
When heated, it decomposes into carbon monoxide (IV) and water.
As a dibasic acid, it forms two types of salts: medium salts - carbonates, acid salts - bicarbonates. They exhibit the general properties of salts. Carbonates and bicarbonates of alkali metals and ammonium are highly soluble in water.
Carbonic acid salts- the compounds are stable, although the acid itself is unstable. They can be obtained by reacting CO2 with solutions of bases or by exchange reactions:
NaOH+CO2=NaHCO3
KHCO3+KOH=K2CO3+H2O
BaCl2+Na2CO3=BaCO3+2NaCl
Carbonates of alkaline earth metals are slightly soluble in water. Hydrocarbonates, on the other hand, are soluble. Hydrocarbonates are formed from carbonates, carbon monoxide (IV) and water:
CaCO3+CO2+H2O=Ca(HCO3)2
When heated, alkali metal carbonates melt without decomposing, and the remaining carbonates, when heated, easily decompose into the oxide of the corresponding metal and CO2:
CaCO3=CaO+CO2
When heated, hydrocarbonates turn into carbonates:
2NaHCO3=Na2CO3+CO2+H2O
Alkali metal carbonates in aqueous solutions have a highly alkaline reaction due to hydrolysis:
Na2CO3+H2O=NaHCO3+NaOH
A qualitative reaction to the carbonate ion C2-3 and bicarbonate HCO-3 is their interaction with stronger acids. The release of carbon monoxide (IV) with a characteristic “boiling” indicates the presence of these ions.
CaCO3+2HCl=CaCl2+CO2+H2O
By passing the released CO2 through lime water, you can observe the solution becoming cloudy due to the formation of calcium carbonate:
Ca(OH)2+CO2=CaCO3+H2O
With prolonged passage of CO2, the solution becomes transparent again due to
formation of bicarbonate: CaCO3+H2O+CO2=Ca(HCO3)2
Continuation. See 21, 22, 23, 24, 25-26, 27-28, 29/2003
6. Carbon subgroup
Know: allotropic modifications of carbon, the dependence of their properties on the structure of the crystal lattice; the most important properties and uses of carbon, carbon oxides, carbonic acid, carbonates, silicon, silicon oxides, silicic acid; composition and production of building materials - glass, cement, concrete, ceramics, conditions for their rational storage and use; qualitative reaction to carbonate ion; methods for detecting carbon dioxide.
Be able to: characterize a subgroup of elements based on the structure of atoms and the position of elements in the periodic table; describe the chemical properties of the studied substances using reaction equations; determine carbonate ion and carbon dioxide in practice; solve combined problems.
Basic concepts: adsorption, desorption, adsorbent, lime water, lime milk, carbides, silicides, silicon anhydride, ceramics.
Security questions
1. What is the valence of carbon in the compounds? Why?
2. What allotropic forms does carbon form?
3. What is the difference between the properties of graphite and diamond? Why are the properties of these substances so different?
4. Why is activated carbon capable of adsorption?
5. What is called adsorption? Where is this property used?
6. What reactions can carbon undergo? Write the reaction equations.
7. What oxides does carbon form?
8. How is the carbon monoxide molecule structured, what type of chemical bond does it have?
9. How can carbon(II) monoxide be obtained? Give the equation of the chemical reaction.
10. What are the physical properties of carbon monoxide?
11. What reactions can carbon monoxide undergo? Give equations for chemical reactions.
12. Where is carbon(II) monoxide used?
13. How does carbon monoxide affect a living organism? How to protect yourself from poisoning with it?
14. How is the carbon dioxide molecule structured, what type of chemical bond does it have?
15. How can you get CO 2? Write an equation for the reaction.
16. What are the physical properties of carbon dioxide?
17. What reactions are possible for carbon dioxide? Give the corresponding reaction equations.
18. How are medium and acidic salts formed in the reactions of CO 2 with alkalis? Write the reaction equations.
19. How to recognize carbon dioxide? Write an equation for the qualitative reaction to CO 2.
20. Why does CO 2 not support combustion and respiration?
21. What is the arrangement of atoms in a carbonic acid molecule?
22. What type of chemical bond between atoms in a carbonic acid molecule?
23. How can you get carbonic acid? Give the reaction equation.
24. How does carbonic acid dissociate? Is it a strong electrolyte?
25. How does sodium carbonate hydrolyze in solution? Write the reaction equation.
26. What is the color of litmus in a solution of carbonic acid? Why?
27. What salts can carbonic acid form? Give examples of formulas of substances.
28. What salts of carbonic acid are found in nature and what are they called?
29. What carbonates are produced in industry?
30. What are the physical properties of carbonic acid salts?
31. How do carbonates behave when heated? Write the reaction equations.
32. What happens to bicarbonates when heated?
33. What other reactions (except decomposition) are possible for carbonates?
34. What is the qualitative reaction to carbonates? Write the reaction equation.
35. Describe the structure of the silicon atom.
36. What are the possible oxidation states of silicon in its compounds?
37. What are the physical properties of silicon?
38. How can you obtain pure silicon? Write an equation for the reaction.
39. What reactions are possible for silicon? Write the reaction equations.
40. How does silicon interact with alkalis? Write an equation for the reaction.
41. Where is silicon used?
42. What oxide does silicon form? In what form does silicon oxide occur in nature?
43. Why is silicon dioxide hard and refractory?
44. What are the chemical properties of silicon dioxide? Write the reaction equations.
45. Where is silicon dioxide used?
46. What is the simplest formula of silicic acid?
47. How can you get silicic acid? Give the reaction equation.
48. What are the physical properties of silicic acid?
49. How are silicates obtained? Write the reaction equations.
50. What are the chemical properties of silicates? Write down reaction equations.
51. Where is silicic acid used?
52. Where are silicates used?
53. What materials does the silicate industry produce?
54. What is the raw material for glass production?
55. How can you change the properties of glass?
56. Where is glass used?
57. Where are ceramic products used?
58. What is the raw material for cement production?
59. Where is cement used?
60. What elements make up the carbon family?
61. How do the properties of elements in the carbon subgroup change with increasing charge of the atomic nucleus? Why?
62. Where are the elements of the carbon family used?
6.1. Solving problems on the topic “Carbon subgroup”
Task 1. When 3.8 g of a mixture of sodium carbonate and sodium bicarbonate was treated with hydrochloric acid, 896 ml of gas was formed
(Well.). What volume of hydrochloric acid (mass fraction - 20%, density - 1.1 g/cm3) was consumed and what was the composition of the initial mixture?
Solution
1. Calculation of the amount of substance:
(CO 2) = 0.896 (l)/22.4 (l/mol) = 0.04 mol.
Let us denote by X the amount of CO 2 gas released in the reaction of Na 2 CO 3 with hydrochloric acid. Then
(CO 2) released during the reaction of NaHCO 3 with HCl is equal to (0.04 - X) mole. Let's write the reaction equations:
2. Let’s make a record to determine the quantitative composition of the mixture:
106X + 84 (0,04 – X) = 3.8, from here X= 0.02 mol;
m(Na 2 CO 3) = 0.02 106 = 2.12 g,
m(NaHCO 3) = 0.02 84 = 1.68 g.
3. Calculate the volume of acid. In the reaction with Na 2 CO 3, 0.04 mol of HCl is consumed, and in the reaction with NaHCO 3 - 0.02 mol of HCl.
Answer. 9.95 ml HCl acid; 2.12 g Na 2 CO 3 and 1.68 g NaHCO 3.
Task 2. What volume of carbon dioxide must be passed (no.) through a solution weighing 80 g with a mass fraction of barium hydroxide dissolved at 5% to obtain barium bicarbonate?
Solution
1. Let’s create the reaction equation:
2. Let’s calculate the amounts of substances of the original compounds that reacted:
m(Ba(OH) 2) = 80 0.05 = 4 g,
(Ba(OH) 2) = 4/171 = 0.0234 mol;
(CO 2) = 2(Ba(OH) 2) = 2 0.0234 = 0.0468 mol.
3. Calculate the volume of gas:
V(CO 2) = 0.0468 22.4 = 1.05 l.
Answer. 1.05 l CO 2.
Task 3. 1 liter of a mixture of carbon oxides (II) and (IV) was passed through lime water. The precipitate that formed was filtered and dried, the mass of the precipitate was 2.45 g. Establish the gas content in the initial mixture as a percentage by volume
(Well.).
Solution
1. Let's write down the reaction equations:

2. Calculate the amount of substance CO 2:
(CO 2) = (CaCO 3) = 2.45/100 = 0.0245 mol.
3. Calculate the volumes and volume fractions () of gases in the mixture:
V(CO 2) = 22.4 0.0245 = 0.5488 l, (CO 2) = 54.88%;
V(SD) = 1 – 0.5488 = 0.4512 l, (SD) = 45.12%.
Answer. Volume fractions (CO 2) = 54.88%; (SD) = 45.12%.
Self-control tasks
1. What substances will carbon(IV) monoxide react with: sodium hydroxide, water, magnesium carbonate, sodium chloride, calcium oxide, copper(II) hydroxide, coal, lime water? Write equations for possible reactions.
2. One test tube contains a solution of sodium carbonate, and the other contains sodium sulfate. A solution of barium chloride was added to each test tube and in both cases a white precipitate formed. How to determine which test tube contains carbonate? Write molecular and ionic reaction equations.
3. Explain redox processes, showing electron transitions using the electron balance method:
4. Write down the reaction equations for the following transformations:
5. When exposed to excess hydrochloric acid on a sample of dolomite MgCO 3 CaCO 3 weighing 50 g, 11.2 liters of carbon dioxide (n.e.) are released. Determine the mass fraction of impurities in this dolomite sample.
Answer. 8%.
6. It is known that when burning coal, 402 kJ/mol is released, and when burning limestone, 180 kJ/mol of heat is absorbed. Using these data, determine the mass of coal (containing 0.98 mass fraction of carbon) required to decompose 1 kg of limestone containing 5% impurities.
Answer. '52
7. 1.68 l of a mixture of carbon(II) and (IV) oxides was passed at room temperature through 50 ml of sodium hydroxide solution with a concentration of 2 mol/l, after which the alkali content in the solution was halved. Determine the composition of the initial mixture of gases in percent by mass and volume.
Answer. (SD) = 33.3%, (SD) = 24.1%;
(CO 2) = 66.7%, (CO 2) = 75.9%.
8. The gas obtained from the complete reduction of 16 g of iron(III) oxide with carbon monoxide is passed through 98.2 ml of a 15% solution of potassium hydroxide (density - 1.14 kg/dm 3). How many liters of carbon monoxide (II) were consumed?
(Well.)? What is the composition and mass of the salt formed?
Answer. 6.72 l CO, 30 g KHSO 3.
7. General properties of metals
Know: position of metals in the periodic table of chemical elements by D.I. Mendeleev; structure and physical properties of metals; occurrence of metals in nature; general chemical properties of metals; types of corrosion and methods of protection against it; electrolysis as a redox process and its application; classification of alloys, composition of some alloys, their properties and applications; the essence and significance of the electrochemical series of metal voltages.
Be able to: characterize metals based on the position of elements in the periodic table and the structure of atoms; characterize the physical properties of metals; draw up reaction equations reflecting the general properties of metals; draw up diagrams and equations for the electrolysis of melts and solutions of salts and alkalis; solve standard and combined problems.
Basic Concepts: metal bond, metal crystal lattice, galvanic cell, electrochemical cell, corrosion, electrolysis, electroextraction, electrolytic refining of metals, electroplating, electroplating, alloys.
Reactions of metals with acids
Active metals can react with acids to release hydrogen (substitution reactions).
Low-active metals do not displace hydrogen from acids.
Security questions
1. What is the importance of metals in human life?
2. What are the structural features of metal atoms?
3. Where are metals located in D.I. Mendeleev’s periodic table of chemical elements?
4. How many outer electrons do the metal atoms of the main and secondary subgroups have?
5. In what forms can metals occur in nature?
6. How can metals be obtained from their compounds?
7. How is the crystal lattice of metals structured?
8. What are the physical properties of metals?
9. How do metal atoms behave in chemical reactions and why?
10. What properties - oxidizing agents or reducing agents - do metals exhibit in chemical reactions?
11. Tell us about the electrochemical voltage series of metals.
12. List the reactions that metals can undergo.
13. How are the chemical activities of metal atoms and metal ions related?
14. Steam s Which metal is deadly? Describe the signs of poisoning.
15. What is metal corrosion and how to protect metal from it?
16. List the alkali metals. Why are they called that?
17. What are the structural features of alkali metal atoms?
18. How can alkali metals be obtained?
19. What are the physical properties of alkali metals?
20. What oxides and peroxides are obtained from the oxidation of alkali metals?
21. What is the oxidation state of the alkali metal in the compound? Why?
22. How is an alkali metal hydride formed? What is the oxidation state of hydrogen in it?
23. How does an alkali metal react with a salt solution?
24. How do alkali metal atoms and ions color the flame?
25. What reactions are characteristic of alkali metals?
26. What chemical bonds do alkali metals form with nonmetals?
27. How does sodium peroxide interact with carbon dioxide?
28. Where are alkali metals used?
29. Which alkali metal is the most active and why?
30. How does superoxide CO 2 interact with CO 2? Write the reaction equation.
7.1. Electrolysis of melts
Cathode
– a reducing agent, the process of accepting electrons by metal cations occurs on it.
Anode
– an oxidizing agent, the process of donating electrons by anions of acidic residues or hydroxide ions occurs on it.
In the case of oxidation of OH – ions, a diagram is drawn up:
4OH – – 4e = 2H 2 O + O 2.
Electrolysis of molten salts.
(Algorithm 30.)
Task 1. Draw up a scheme for the electrolysis of molten sodium bromide.
Task 2. Draw up a scheme for the electrolysis of molten sodium sulfate.
Electrolysis of alkali melts.
(Algorithm 31.)
Task 1. Draw up a scheme for the electrolysis of molten sodium hydroxide.
7.2. Electrolysis of solutions
Electrolysis is the redox process that occurs at the electrodes when an electric current is passed through the electrolyte. During electrolysis, the cathode is a reducing agent, because it gives up electrons, and the anode is an oxidizing agent, because it accepts electrons from anions.
To select the most likely process at the cathode and anode during the electrolysis of solutions using an inert (insoluble) anode (for example, graphite, coal, platinum, iridium), use the following rules.
1. The following are formed at the anode:
a) during electrolysis of solutions containing F – anions, –
, , , OH – , – O 2 ;
b) during the oxidation of the anions Cl – , Br – , I – – Cl 2 , Br 2 , I 2 , respectively.
2. The following are formed at the cathode:
a) during the electrolysis of solutions containing ions located in the voltage series to the left of Al 3+, – H 2;
b) if the ions are located in the voltage series to the right of hydrogen - metals;
c) if the ions are located in the voltage range between Al 3+ and H +, then competing processes can occur at the cathode - the reduction of both metals and hydrogen;
d) if an aqueous solution contains cations of various metals, then their reduction occurs in order of decreasing the value of the standard electrode potential (from right to left along the series of metal voltages).
In the case of using an active (soluble) anode (made of copper, silver, zinc, nickel, cadmium), the anode itself undergoes oxidation (dissolves) and at the cathode, in addition to metal cations, salts and hydrogen ions, metal cations obtained by dissolving the anode are reduced.
It is convenient to compare the reduction properties of metals using the electrochemical voltage series, which includes hydrogen. The reducing ability of elements in this series decreases from left to right, and the oxidizing ability of the corresponding cations increases in the same direction.
Electrolysis of an aqueous salt solution.
(Algorithm 32.)
Task 1. Draw up a scheme for the electrolysis of an aqueous solution of sodium chloride using inert electrodes.
Task 2. Draw up a scheme for the electrolysis of an aqueous solution of copper(II) sulfate using inert electrodes.
Electrolysis of aqueous alkali solution.
(Algorithm 33.)
Task 1. Draw up a scheme for the electrolysis of an aqueous solution of sodium hydroxide.
Self-control tasks
1. Make electrolysis schemes:
a) melts of calcium chloride, potassium hydroxide, lithium sulfate;
b) aqueous solutions of magnesium chloride, potassium sulfate, mercury(II) nitrate.
2. What reactions are practically feasible:
a) Cu + HCl ... ;
b) Mg + H 2 SO 4 (diluted) ...;
c) Zn + Pb(NO 3) 2 ...;
d) Cu + ZnCl 2 ...;
e) Ca + H 2 O ...;
e) Fe + Cl 2 ... ?
3. There is a copper rivet on the steel cover. What will break first - the cover or the rivet? Why?
4. There is an iron product covered with a protective film of tin (tinned iron). What will happen when such a product is heated in air? Write the equations for the reactions that occur.
5. What volume of hydrogen (n.u.) will be released when 20 g of a product made from an alloy of sodium, potassium and copper in a mass ratio of 1:1:2 is immersed in water?
Answer. 3.86 l.
6. Calculate the mass of a 9.8% sulfuric acid solution that will be required to dissolve four zinc granules if the mass of each granule is 0.2 g.
Answer. 12.3 g.
7. Calculate what the mass fraction of potassium hydroxide in the solution will be if potassium metal weighing 3.9 g is dissolved in 80 ml of water.
Answer. 6.68%.
8. During the electrolysis of a certain metal sulfate, 176 ml of oxygen (n.o.) was released at the anode, and 1 g of metal was released at the cathode during the same time. What metal sulfate was taken?
Answer. CuSO4.
9. An iron plate weighing 18 g is immersed in a solution of copper(II) sulfate. When it was coated with copper, its mass became 18.2 g. What mass of iron went into solution?
Answer. 1.4 g.
10. An iron plate weighing 5 g is immersed for some time in 50 ml of a 15% solution of copper(II) sulfate, the density of which is 1.12 g/cm 3 . After the plate was removed, its mass was found to be 5.16 g. What is the mass of copper(II) sulfate in the remaining solution?
Answer. 5.2 g.
Answers to tasks for self-control
6.1. Solving problems on the topic “Carbon subgroup”


Soda, volcano, Venus, refrigerator - what do they have in common? Carbon dioxide. We have collected for you the most interesting information about one of the most important chemical compounds on Earth.
What is carbon dioxide
Carbon dioxide is known mainly in its gaseous state, i.e. as carbon dioxide with the simple chemical formula CO2. In this form, it exists under normal conditions - at atmospheric pressure and “ordinary” temperatures. But at increased pressure, above 5,850 kPa (such as, for example, the pressure at a sea depth of about 600 m), this gas turns into liquid. And when strongly cooled (minus 78.5°C), it crystallizes and becomes so-called dry ice, which is widely used in trade for storing frozen foods in refrigerators.
Liquid carbon dioxide and dry ice are produced and used in human activities, but these forms are unstable and easily disintegrate.
But carbon dioxide gas is ubiquitous: it is released during the respiration of animals and plants and is an important part of the chemical composition of the atmosphere and ocean.
Properties of carbon dioxide
Carbon dioxide CO2 is colorless and odorless. Under normal conditions it has no taste. However, if you inhale high concentrations of carbon dioxide, you may experience a sour taste in your mouth, caused by the carbon dioxide dissolving on mucous membranes and in saliva, forming a weak solution of carbonic acid.
By the way, it is the ability of carbon dioxide to dissolve in water that is used to make carbonated water. Lemonade bubbles are the same carbon dioxide. The first apparatus for saturating water with CO2 was invented back in 1770, and already in 1783, the enterprising Swiss Jacob Schweppes began industrial production of soda (the Schweppes brand still exists).
Carbon dioxide is 1.5 times heavier than air, so it tends to “settle” in its lower layers if the room is poorly ventilated. The “dog cave” effect is known, where CO2 is released directly from the ground and accumulates at a height of about half a meter. An adult, entering such a cave, at the height of his growth does not feel the excess of carbon dioxide, but dogs find themselves directly in a thick layer of carbon dioxide and are poisoned.
CO2 does not support combustion, which is why it is used in fire extinguishers and fire suppression systems. The trick of extinguishing a burning candle with the contents of a supposedly empty glass (but in fact carbon dioxide) is based precisely on this property of carbon dioxide.
Carbon dioxide in nature: natural sources
Carbon dioxide is formed in nature from various sources:
- Respiration of animals and plants.
Every schoolchild knows that plants absorb carbon dioxide CO2 from the air and use it in the processes of photosynthesis. Some housewives try to make up for shortcomings with an abundance of indoor plants. However, plants not only absorb, but also release carbon dioxide in the absence of light - this is part of the respiration process. Therefore, a jungle in a poorly ventilated bedroom is not a good idea: CO2 levels will rise even more at night. - Volcanic activity.
Carbon dioxide is part of volcanic gases. In areas with high volcanic activity, CO2 can be released directly from the ground - from cracks and fissures called mofets. The concentration of carbon dioxide in valleys with mofets is so high that many small animals die when they get there. - Decomposition of organic matter.
Carbon dioxide is formed during the combustion and decay of organic matter. Large natural emissions of carbon dioxide accompany forest fires.
Carbon dioxide is “stored” in nature in the form of carbon compounds in minerals: coal, oil, peat, limestone. Huge reserves of CO2 are found in dissolved form in the world's oceans.

The release of carbon dioxide from an open reservoir can lead to a limnological catastrophe, as happened, for example, in 1984 and 1986. in lakes Manoun and Nyos in Cameroon. Both lakes were formed on the site of volcanic craters - now they are extinct, but in the depths the volcanic magma still releases carbon dioxide, which rises to the waters of the lakes and dissolves in them. As a result of a number of climatic and geological processes, the concentration of carbon dioxide in waters exceeded a critical value. A huge amount of carbon dioxide was released into the atmosphere, which went down the mountain slopes like an avalanche. About 1,800 people became victims of limnological disasters on Cameroonian lakes.
Artificial sources of carbon dioxide
The main anthropogenic sources of carbon dioxide are:
- industrial emissions associated with combustion processes;
- road transport.
Despite the fact that the share of environmentally friendly transport in the world is growing, the vast majority of the world's population will not soon have the opportunity (or desire) to switch to new cars.
Active deforestation for industrial purposes also leads to an increase in the concentration of carbon dioxide CO2 in the air.
CO2 is one of the end products of metabolism (the breakdown of glucose and fats). It is secreted in the tissues and transported by hemoglobin to the lungs, through which it is exhaled. The air exhaled by humans contains about 4.5% carbon dioxide (45,000 ppm) - 60-110 times more than in the air inhaled.
Carbon dioxide plays a large role in regulating blood flow and respiration. An increase in CO2 levels in the blood causes the capillaries to dilate, allowing more blood to pass through, which delivers oxygen to the tissues and removes carbon dioxide.

The respiratory system is also stimulated by an increase in carbon dioxide, and not by a lack of oxygen, as it might seem. In reality, the lack of oxygen is not felt by the body for a long time, and it is quite possible that in rarefied air a person will lose consciousness before he feels the lack of air. The stimulating property of CO2 is used in artificial respiration machines: where carbon dioxide is mixed with oxygen to “start” the respiratory system.
Carbon dioxide and us: why CO2 is dangerous
Carbon dioxide is necessary for the human body just like oxygen. But just like with oxygen, an excess of carbon dioxide harms our well-being.
A high concentration of CO2 in the air leads to intoxication of the body and causes a state of hypercapnia. With hypercapnia, a person experiences difficulty breathing, nausea, headache, and may even lose consciousness. If the carbon dioxide content does not decrease, then oxygen starvation occurs. The fact is that both carbon dioxide and oxygen move throughout the body on the same “transport” - hemoglobin. Normally, they “travel” together, attaching to different places on the hemoglobin molecule. However, increased concentrations of carbon dioxide in the blood reduce the ability of oxygen to bind to hemoglobin. The amount of oxygen in the blood decreases and hypoxia occurs.
Such unhealthy consequences for the body occur when inhaling air with a CO2 content of more than 5,000 ppm (this can be the air in mines, for example). To be fair, in ordinary life we practically never encounter such air. However, a much lower concentration of carbon dioxide does not have the best effect on health.
According to some findings, even 1,000 ppm CO2 causes fatigue and headaches in half of the subjects. Many people begin to feel stuffiness and discomfort even earlier. With a further increase in carbon dioxide concentration to 1,500 – 2,500 ppm critically, the brain is “lazy” to take the initiative, process information and make decisions.
And if a level of 5,000 ppm is almost impossible in everyday life, then 1,000 and even 2,500 ppm can easily be part of the reality of modern man. Ours showed that in rarely ventilated school classrooms, CO2 levels remain above 1,500 ppm much of the time, and sometimes jump above 2,000 ppm. There is every reason to believe that the situation is similar in many offices and even apartments.
Physiologists consider 800 ppm to be a safe level of carbon dioxide for human well-being.
Another study found a link between CO2 levels and oxidative stress: the higher the carbon dioxide level, the more we suffer from oxidative stress, which damages our body's cells.
Carbon dioxide in the Earth's atmosphere
There is only about 0.04% CO2 in the atmosphere of our planet (this is approximately 400 ppm), and more recently it was even less: carbon dioxide crossed the 400 ppm mark only in the fall of 2016. Scientists attribute the rise in CO2 levels in the atmosphere to industrialization: in the mid-18th century, on the eve of the Industrial Revolution, it was only about 270 ppm.