Mobile electronics are becoming more accessible and widespread every year, if not month. Here you will find laptops, PDAs, digital cameras, mobile phones, and a host of other useful and not so useful devices. And all these devices are constantly acquiring new features, more powerful processors, larger color screens, wireless communications, while at the same time decreasing in size. But, unlike semiconductor technologies, power technologies for this entire mobile menagerie are not progressing by leaps and bounds.
Conventional batteries and rechargeable batteries are becoming clearly insufficient to power the latest advances in the electronics industry for any significant period of time. And without reliable and capacious batteries, the whole point of mobility and wirelessness is lost. So the computer industry is working more and more actively on the problem alternative sources nutrition. And the most promising direction here today is fuel cells.
The basic operating principle of fuel cells was discovered by British scientist Sir William Grove in 1839. He is known as the father of the "fuel cell". William Grove generated electricity by altering to extract hydrogen and oxygen. Having disconnected the battery from the electrolytic cell, Grove was surprised to find that the electrodes began to absorb the released gas and generate current. Opening a process electrochemical "cold" combustion of hydrogen became a significant event in the energy industry, and subsequently such famous electrochemists as Ostwald and Nernst played a major role in the development of the theoretical foundations and practical implementation of fuel cells and predicted a great future for them.
Myself term "fuel cell" appeared later - it was proposed in 1889 by Ludwig Mond and Charles Langer, who were trying to create a device for generating electricity from air and coal gas.
During normal combustion in oxygen, oxidation of organic fuel occurs, and the chemical energy of the fuel is inefficiently converted into thermal energy. But it turned out to be possible to carry out the oxidation reaction, for example, of hydrogen with oxygen, in an electrolyte environment and, in the presence of electrodes, obtain electricity. For example, by supplying hydrogen to an electrode located in an alkaline medium, we obtain electrons:
2H2 + 4OH- → 4H2O + 4e-
which, passing through the external circuit, arrive at the opposite electrode, to which oxygen flows and where the reaction takes place: 4e- + O2 + 2H2O → 4OH-
It can be seen that the resulting reaction 2H2 + O2 → H2O is the same as during conventional combustion, but in a fuel cell, or otherwise - in electrochemical generator, the result is electric current with great efficiency and partially heat. Note that coal, carbon monoxide, alcohols, hydrazine, and others can also be used as fuel in fuel cells. organic matter, and as oxidizing agents - air, hydrogen peroxide, chlorine, bromine, Nitric acid etc.
The development of fuel cells continued vigorously both abroad and in Russia, and then in the USSR. Among the scientists who made a great contribution to the study of fuel cells, we note V. Jaco, P. Yablochkov, F. Bacon, E. Bauer, E. Justi, K. Cordesh. In the middle of the last century, a new assault on fuel cell problems began. This is partly due to the emergence of new ideas, materials and technologies as a result of defense research.
One of the scientists who made a major step in the development of fuel cells was P. M. Spiridonov. Hydrogen-oxygen elements of Spiridonov gave a current density of 30 mA/cm2, which was considered a great achievement at that time. In the forties, O. Davtyan created an installation for the electrochemical combustion of generator gas obtained by gasification of coal. For each cubic meter of element volume, Davtyan received 5 kW of power.
It was first solid electrolyte fuel cell. It had high efficiency, but over time the electrolyte became unusable and needed to be changed. Subsequently, Davtyan, in the late fifties, created a powerful installation that drives the tractor. In the same years, the English engineer T. Bacon designed and built a battery of fuel cells with a total power of 6 kW and an efficiency of 80%, running on pure hydrogen and oxygen, but the power-to-weight ratio of the battery turned out to be too small - such elements were unsuitable for practical application and too expensive.
In subsequent years, the time for loners passed. The creators of spacecraft became interested in fuel cells. Since the mid-60s, millions of dollars have been invested in fuel cell research. The work of thousands of scientists and engineers allowed us to reach a new level, and in 1965. fuel cells were tested in the USA on the Gemini 5 spacecraft, and later on the Apollo spacecraft for flights to the Moon and the Shuttle program.
In the USSR, fuel cells were developed at NPO Kvant, also for use in space. In those years, new materials had already appeared - solid polymer electrolytes based on ion exchange membranes, new types of catalysts, electrodes. Still, the operating current density was small - in the range of 100-200 mA/cm2, and the platinum content on the electrodes was several g/cm2. There were many problems related to durability, stability, and safety.
The next stage of rapid development of fuel cells began in the 90s. last century and continues to this day. It is caused by the need for new efficient energy sources in connection, on the one hand, with the global environmental problem of increasing greenhouse gas emissions from the combustion of fossil fuels and, on the other hand, with the depletion of reserves of such fuel. Since in a fuel cell the final product of hydrogen combustion is water, they are considered the cleanest in terms of environmental impact. The main problem is just finding an effective and inexpensive way obtaining hydrogen.
Billions of dollars in financial investments in the development of fuel cells and hydrogen generators should lead to a technological breakthrough and make their use in everyday life a reality: in cells for cell phones, in cars, in power plants. Already, such automobile giants as Ballard, Honda, Daimler Chrysler, and General Motors are demonstrating cars and buses powered by fuel cells with a power of 50 kW. A number of companies have developed demonstration power plants using fuel cells with solid oxide electrolyte with a power of up to 500 kW. But, despite a significant breakthrough in improving the characteristics of fuel cells, many problems related to their cost, reliability, and safety still need to be solved.
In a fuel cell, unlike batteries and accumulators, both fuel and oxidizer are supplied to it from the outside. The fuel cell only mediates the reaction and, under ideal conditions, could operate virtually forever. The beauty of this technology is that the cell actually burns fuel and directly converts the released energy into electricity. When fuel is directly burned, it is oxidized by oxygen, and the heat released is used to perform useful work.
In a fuel cell, as in batteries, the reactions of fuel oxidation and oxygen reduction are spatially separated, and the “combustion” process occurs only if the cell supplies current to the load. It's just like diesel electric generator, only without diesel and generator. And also without smoke, noise, overheating and with much higher efficiency. The latter is explained by the fact that, firstly, there are no intermediate mechanical devices and, secondly, the fuel cell is not a heat engine and, as a result, does not obey Carnot’s law (that is, its efficiency is not determined by the temperature difference).
Oxygen is used as an oxidizing agent in fuel cells. Moreover, since there is enough oxygen in the air, there is no need to worry about supplying an oxidizing agent. As for fuel, it is hydrogen. So, the reaction takes place in the fuel cell:
2H2 + O2 → 2H2O + electricity + heat.
The result is useful energy and water vapor. The simplest in its structure is proton exchange membrane fuel cell(see Figure 1). It works as follows: hydrogen entering the element is decomposed under the action of a catalyst into electrons and positively charged hydrogen ions H+. Then a special membrane comes into play, playing the role of an electrolyte in a conventional battery. Due to its chemical composition it allows protons to pass through but retains electrons. Thus, the electrons accumulated on the anode create an excess negative charge, and the hydrogen ions create a positive charge on the cathode (the voltage across the element is about 1V).
To create high power, a fuel cell is assembled from many cells. If you connect an element to a load, electrons will flow through it to the cathode, creating a current and completing the process of oxidation of hydrogen with oxygen. Platinum microparticles deposited on carbon fiber are usually used as a catalyst in such fuel cells. Due to its structure, such a catalyst allows gas and electricity to pass through well. The membrane is usually made from the sulfur-containing polymer Nafion. The thickness of the membrane is tenths of a millimeter. During the reaction, of course, heat is also released, but not so much of it, so the operating temperature is maintained in the region of 40-80°C.
Fig.1. Operating principle of a fuel cell
There are other types of fuel cells, mainly differing in the type of electrolyte used. Almost all of them require hydrogen as fuel, so the logical question arises: where to get it. Of course, it would be possible to use compressed hydrogen from cylinders, but problems immediately arise associated with the transportation and storage of this highly flammable gas under high pressure. Of course, hydrogen can be used in bound form, as in metal hydride batteries. But the task of extracting and transporting it still remains, because the infrastructure for hydrogen refueling does not exist.
However, there is also a solution here - liquid hydrocarbon fuel can be used as a source of hydrogen. For example, ethyl or methyl alcohol. True, this requires a special additional device - a fuel converter, which at high temperatures (for methanol it will be about 240 ° C) converts alcohols into a mixture of gaseous H2 and CO2. But in this case, it is already more difficult to think about portability - such devices are good to use as stationary devices, but for compact mobile equipment you need something less bulky.
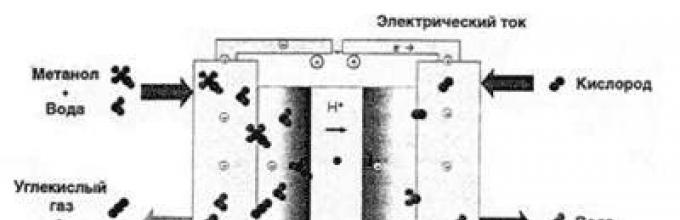
And here we come to exactly the device that almost everyone is developing with terrible force. largest producers electronics - methanol fuel cell(Figure 2).

Fig.2. Operating principle of a methanol fuel cell
The fundamental difference between hydrogen and methanol fuel cells is the catalyst used. The catalyst in a methanol fuel cell allows protons to be removed directly from the alcohol molecule. Thus, the issue with fuel is resolved - methyl alcohol is mass-produced for the chemical industry, it is easy to store and transport, and to charge a methanol fuel cell it is enough to simply replace the fuel cartridge. True, there is one significant disadvantage - methanol is toxic. In addition, the efficiency of a methanol fuel cell is significantly lower than that of a hydrogen one.

Rice. 3. Methanol fuel cell
The most tempting option is to use ethyl alcohol as fuel, since the production and distribution of alcoholic beverages of any composition and strength is well established throughout the globe. However, the efficiency of ethanol fuel cells, unfortunately, is even lower than that of methanol ones.
As has already been noted over many years of development in the field of fuel cells, Various types fuel cells. Fuel cells are classified by electrolyte and fuel type.
1. Solid polymer hydrogen-oxygen electrolyte.
2. Solid polymer methanol fuel cells.
3. Alkaline electrolyte cells.
4. Phosphoric acid fuel cells.
5. Fuel elements based on molten carbonates.
6. Solid oxide fuel cells.
Ideally, the efficiency of fuel cells is very high, but in real conditions there are losses associated with nonequilibrium processes, such as: ohmic losses due to the specific conductivity of the electrolyte and electrodes, activation and concentration polarization, and diffusion losses. As a result, part of the energy generated in fuel cells is converted into heat. The efforts of specialists are aimed at reducing these losses.
The main source of ohmic losses, as well as the reason for the high price of fuel cells, are perfluorinated sulfonic cation exchange membranes. The search is now underway for alternative, cheaper proton-conducting polymers. Since the conductivity of these membranes (solid electrolytes) reaches an acceptable value (10 Ohm/cm) only in the presence of water, the gases supplied to the fuel cell must be additionally humidified in a special device, which also increases the cost of the system. Catalytic gas diffusion electrodes mainly use platinum and some other noble metals, and so far no replacement has been found for them. Although the platinum content in fuel cells is several mg/cm2, for large batteries its amount reaches tens of grams.
When designing fuel cells, much attention is paid to the heat removal system, since at high current densities (up to 1A/cm2) the system self-heats. For cooling, water is used circulating in the fuel cell through special channels, and at low powers - air blowing.
So, a modern electrochemical generator system, in addition to the fuel cell battery itself, is “overgrown” with many auxiliary devices, such as: pumps, a compressor for supplying air, injecting hydrogen, a gas humidifier, a cooling unit, a gas leakage monitoring system, a DC-AC converter, a control processor etc. All this leads to the fact that the cost of a fuel cell system in 2004-2005 was 2-3 thousand $/kW. According to experts, fuel cells will become available for use in transport and stationary power plants at a price of $50-100/kW.
To introduce fuel cells into daily life, along with cheaper components, we must expect new original ideas and approaches. In particular, great hopes are pinned on the use of nanomaterials and nanotechnologies. For example, several companies have recently announced the creation of ultra-efficient catalysts, in particular for oxygen electrodes, based on clusters of nanoparticles from various metals. In addition, there have been reports of membraneless fuel cell designs in which liquid fuel (such as methanol) is fed into the fuel cell along with an oxidizer. Also interesting is the developing concept of biofuel cells operating in polluted waters and consuming dissolved air oxygen as an oxidizer, and organic impurities as fuel.
According to experts, fuel cells will enter the mass market in the coming years. Indeed, one after another, developers overcome technical problems, report successes and present prototypes of fuel cells. For example, Toshiba demonstrated a finished prototype of a methanol fuel cell. It has a size of 22x56x4.5mm and produces a power of about 100mW. One refill of 2 cubes of concentrated (99.5%) methanol is enough for 20 hours of operation of the MP3 player. Toshiba has released a commercial fuel cell to power mobile phones. Again, the same Toshiba demonstrated a cell for powering laptops measuring 275x75x40mm, allowing the computer to operate for 5 hours on a single charge.
Another Japanese company, Fujitsu, is not far behind Toshiba. In 2004, she also introduced an element that operates in a 30% aqueous solution of methanol. This fuel cell operated on one 300 ml charge for 10 hours and produced a power of 15 W.
Casio is developing a fuel cell in which methanol is first converted into a mixture of H2 and CO2 gases in a miniature fuel converter, and then fed into the fuel cell. During the demonstration, the Casio prototype powered a laptop for 20 hours.
Samsung also made its mark in the field of fuel cells - in 2004, it demonstrated its 12 W prototype designed to power a laptop. In general, Samsung plans to use fuel cells primarily in fourth-generation smartphones.
It must be said that Japanese companies generally took a very thorough approach to the development of fuel cells. Back in 2003, companies such as Canon, Casio, Fujitsu, Hitachi, Sanyo, Sharp, Sony and Toshiba joined forces to develop a single fuel cell standard for laptops, mobile phones, PDAs and other electronic devices. American companies, of which there are also many in this market, mostly work under contracts with the military and develop fuel cells for the electrification of American soldiers.
The Germans are not far behind - the Smart Fuel Cell company sells fuel cells to power a mobile office. The device is called Smart Fuel Cell C25, has dimensions of 150x112x65mm and can deliver up to 140 watt-hours per fill. This is enough to power the laptop for approximately 7 hours. Then the cartridge can be replaced and you can continue working. The size of the methanol cartridge is 99x63x27 mm, and it weighs 150g. The system itself weighs 1.1 kg, so it cannot be called completely portable, but it is still a completely complete and convenient device. The company is also developing a fuel module to power professional video cameras.
In general, fuel cells have almost entered the mobile electronics market. Manufacturers still have to solve the last technical problems before starting mass production.
First, it is necessary to resolve the issue of miniaturization of fuel cells. After all, the smaller the fuel cell, the less power it can produce - so new catalysts and electrodes are constantly being developed that make it possible to maximize the working surface with small sizes. This is where the latest developments in the field of nanotechnology and nanomaterials (for example, nanotubes) come in very handy. Again, to miniaturize the piping of elements (fuel and water pumps, cooling and fuel conversion systems), achievements of microelectromechanics are increasingly being used.
The second important problem that needs to be addressed is price. After all, very expensive platinum is used as a catalyst in most fuel cells. Again, some of the manufacturers are trying to make the most of already well-established silicon technologies.
As for other areas of use of fuel cells, fuel cells have already become quite firmly established there, although they have not yet become mainstream either in the energy sector or in transport. Already, many car manufacturers have presented their concept cars powered by fuel cells. Fuel cell buses are running in several cities around the world. Canadian Ballard Power Systems produces a range of stationary generators with a capacity from 1 to 250 kW. At the same time, kilowatt generators are designed to immediately supply one apartment with electricity, heat and hot water.
The US has several initiatives aimed at developing hydrogen fuel cells, infrastructure and technology to make fuel cell vehicles practical and fuel efficient by 2020. More than one billion dollars have been allocated for these purposes.
Fuel cells generate electricity quietly and efficiently, without pollution environment. Unlike energy sources that use fossil fuels, the byproducts of fuel cells are heat and water. How it works?
In this article we will briefly look at each of the existing fuel technologies today, as well as talk about the design and operation of fuel cells, and compare them with other forms of energy production. We'll also discuss some of the obstacles researchers face in making fuel cells practical and affordable for consumers.
Fuel cells are electrochemical energy conversion devices. A fuel cell converts chemicals, hydrogen and oxygen, into water, generating electricity in the process.
Another electrochemical device that we are all very familiar with is the battery. The battery has everything you need chemical elements inside itself and turns these substances into electricity. This means that the battery eventually dies and you either throw it away or charge it again.
In a fuel cell, chemicals are continually fed into it so that it never “dies.” Electricity will be generated as long as chemicals enter the element. Most fuel cells in use today use hydrogen and oxygen.
Hydrogen is the most common element in our Galaxy. However, hydrogen practically does not exist on Earth in its elemental form. Engineers and scientists must extract pure hydrogen from hydrogen compounds, including fossil fuels or water. To extract hydrogen from these compounds, you need to expend energy in the form of heat or electricity.
Invention of fuel cells
Sir William Grove invented the first fuel cell in 1839. Grove knew that water could be split into hydrogen and oxygen by passing an electric current through it (a process called electrolysis). He suggested that in reverse order it would be possible to obtain electricity and water. He created a primitive fuel cell and called it gas galvanic battery. After experimenting with his new invention, Grove proved his hypothesis. Fifty years later, scientists Ludwig Mond and Charles Langer coined the term fuel cells when trying to build a practical model for generating electricity.
The fuel cell will compete with many other energy conversion devices, including gas turbines in urban power plants, internal combustion engines in cars, and all kinds of batteries. Internal combustion engines, like gas turbines, burn different types of fuel and use the pressure created by the expansion of gases to perform mechanical work. Batteries convert chemical energy into electrical energy when needed. Fuel cells must perform these tasks more efficiently.
The fuel cell provides DC (direct current) voltage which can be used to power electric motors, lights and other electrical appliances.
There are several different types of fuel cells, each using different chemical processes. Fuel cells are usually classified according to their operating temperature And typeelectrolyte, which they use. Some types of fuel cells are well suited for use in stationary power plants. Others may be useful for small portable devices or for powering cars. The main types of fuel cells include:
Polymer exchange membrane fuel cell (PEMFC)
PEMFC is considered as the most likely candidate for transport applications. PEMFC has both high power and relatively low operating temperature (ranging from 60 to 80 degrees Celsius). Low operating temperatures mean fuel cells can quickly warm up to begin generating electricity.
Solid oxide fuel cell (SOFC)
These fuel cells are most suitable for large stationary power generators that could power factories or cities. This type of fuel cell operates at very high temperatures (700 to 1000 degrees Celsius). High temperature poses a reliability problem because some fuel cells can fail after a few on-off cycles. However, solid oxide fuel cells are very stable during continuous operation. In fact, SOFCs have demonstrated the longest operating life of any fuel cell under certain conditions. The high temperature also has the advantage that the steam produced by the fuel cells can be sent to turbines and generate more electricity. This process is called cogeneration of heat and electricity and improves overall system efficiency.
Alkaline fuel cell (AFC)
It is one of the oldest designs for fuel cells, having been in use since the 1960s. AFCs are very susceptible to contamination as they require pure hydrogen and oxygen. In addition, they are very expensive, so this type of fuel cell is unlikely to be put into mass production.
Molten-carbonate fuel cell (MCFC)
Like SOFCs, these fuel cells are also best suited for large stationary power plants and generators. They operate at 600 degrees Celsius so they can generate steam, which in turn can be used to generate even more energy. They have a lower operating temperature than solid oxide fuel cells, which means they do not require such heat-resistant materials. This makes them a little cheaper.
Phosphoric-acid fuel cell (PAFC)
Phosphoric acid fuel cell has potential for use in small stationary power systems. It operates at a higher temperature than a polymer exchange membrane fuel cell, so it takes longer to warm up, making it unsuitable for use in automobiles.
Direct methanol fuel cell (DMFC)
Methanol fuel cells are comparable to PEMFC in terms of operating temperature, but are not as efficient. In addition, DMFCs require quite large quantity platinum acts as a catalyst, which makes these fuel cells expensive.
Fuel cell with polymer exchange membrane
Polymer exchange membrane fuel cell (PEMFC) is one of the most promising fuel cell technologies. PEMFC uses one of the simplest reactions of any fuel cell. Let's look at what it consists of.
1. A node – negative terminal of the fuel cell. It conducts electrons that are released from hydrogen molecules, after which they can be used in an external circuit. It has engraved channels through which hydrogen gas is distributed evenly over the surface of the catalyst.
2.TO athode - the positive terminal of the fuel cell also has channels for distributing oxygen over the surface of the catalyst. It also conducts electrons back from the catalyst's external circuit, where they can combine with hydrogen and oxygen ions to form water.
3.Electrolyte-proton exchange membrane. This is a specially treated material that conducts only positively charged ions and blocks electrons. With PEMFC, the membrane must be hydrated in order to function properly and remain stable.
4. Catalyst is a special material that promotes the reaction of oxygen and hydrogen. It is typically made from platinum nanoparticles applied very thinly to carbon paper or fabric. The catalyst has a surface structure such that maximum surface area of the platinum can be exposed to hydrogen or oxygen.

The figure shows hydrogen gas (H2) entering the fuel cell under pressure from the anode side. When an H2 molecule comes into contact with platinum on the catalyst, it splits into two H+ ions and two electrons. The electrons pass through the anode, where they are used in external circuitry (doing useful work, such as turning a motor), and return to the cathode side of the fuel cell.
Meanwhile, on the cathode side of the fuel cell, oxygen (O2) from the air passes through the catalyst where it forms two oxygen atoms. Each of these atoms has a strong negative charge. This negative charge attracts two H+ ions across the membrane, where they combine with an oxygen atom and two electrons coming from the external circuit to form a water molecule (H2O).
This reaction in a single fuel cell produces only about 0.7 Volts. To raise the voltage to a reasonable level, many individual fuel cells must be combined to form a fuel cell stack. Bipolar plates are used to connect one fuel cell to another and undergo oxidation to reduce potential. The big problem with bipolar plates is their stability. Metal bipolar plates can be corroded, and by-products (iron and chromium ions) reduce the efficiency of the fuel cell membranes and electrodes. Therefore, low temperature fuel cells use light metals, graphite and composites of carbon and thermoset (thermoset is a kind of plastic that remains solid even when exposed to high temperatures) in the form of bipolar sheet material.
Fuel cell efficiency
Reducing pollution is one of the main goals of a fuel cell. By comparing a car powered by a fuel cell to a car powered by a gasoline engine and a car powered by a battery, you can see how fuel cells could improve the efficiency of cars.
Since all three types of cars have many of the same components, we will ignore this part of the car and compare useful actions to the point where mechanical energy is produced. Let's start with the fuel cell car.
If the fuel cell is powered by pure hydrogen, its efficiency can be up to 80 percent. Thus, it converts 80 percent of the energy content of hydrogen into electricity. However, we still have to convert electrical energy into mechanical work. This is achieved by an electric motor and an inverter. The efficiency of the motor + inverter is also approximately 80 percent. This gives an overall efficiency of approximately 80*80/100=64 percent. Honda's FCX concept vehicle reportedly has 60 percent energy efficiency.
If the fuel source is not in the form of pure hydrogen, then the vehicle will also need a reformer. Reformers convert hydrocarbon or alcohol fuels into hydrogen. They generate heat and produce CO and CO2 in addition to hydrogen. They use various devices to purify the resulting hydrogen, but this purification is insufficient and reduces the efficiency of the fuel cell. Therefore, the researchers decided to concentrate on fuel cells for Vehicle, running on pure hydrogen, despite the challenges associated with hydrogen production and storage.
Efficiency of a gasoline engine and a battery-electric car
The efficiency of a car powered by gasoline is surprisingly low. All heat that is exhausted or absorbed by the radiator is wasted energy. The engine also uses a lot of power to drive the various pumps, fans and generators that keep it running. Thus, the overall efficiency of a gasoline automobile engine is approximately 20 percent. Thus, only about 20 percent of gasoline's thermal energy content is converted into mechanical work.
A battery-powered electric vehicle has fairly high efficiency. The battery is approximately 90 percent efficient (most batteries generate some heat or require heating), and the motor + inverter is approximately 80 percent efficient. This gives an overall efficiency of approximately 72 percent.
But that's not all. In order for an electric car to move, electricity must first be generated somewhere. If it was a power plant that used a fossil fuel combustion process (rather than nuclear, hydroelectric, solar or wind power), then only approximately 40 percent of the fuel consumed by the power plant was converted into electricity. Plus, the process of charging a car requires converting alternating current (AC) power to direct current (DC) power. This process has an efficiency of approximately 90 percent.
Now, if we look at the whole cycle, the efficiency of an electric vehicle is 72 percent for the vehicle itself, 40 percent for the power plant, and 90 percent for charging the vehicle. This gives an overall efficiency of 26 percent. Overall efficiency varies significantly depending on which power plant is used to charge the battery. If the car's electricity is generated by a hydroelectric power plant, for example, the electric car's efficiency will be approximately 65 percent.
Scientists are researching and improving designs to continue improving the efficiency of the fuel cell. One new approach would be to combine fuel cell and battery-powered vehicles. A concept vehicle powered by a hybrid powertrain powered by a fuel cell is being developed. It uses a lithium battery to power the car while the fuel cell recharges the battery.
Fuel cell vehicles are potentially as efficient as a battery-powered car that is charged from a power plant that does not use fossil fuels. But achieving this potential in a practical and accessible way can be difficult.
Why use fuel cells?
The main reason is everything related to oil. America must import nearly 60 percent of its oil. By 2025, imports are expected to rise to 68%. Americans use two-thirds of oil daily for transportation. Even if every car on the street were a hybrid car, by 2025 the US would still need to use the same amount of oil that Americans consumed in 2000. In fact, America consumes a quarter of all the oil produced in the world, although only 4.6% of the world's population lives here.
Experts expect oil prices to continue rising over the next few decades as cheaper sources dwindle. Oil companies must develop oil fields in increasingly difficult conditions, which will increase oil prices.
Concerns extend far beyond economic security. A lot of money coming from oil sales is spent on supporting international terrorism, radical political parties, unstable situation in oil-producing regions.
The use of oil and other fossil fuels for energy produces pollution. It is best for everyone to find an alternative to burning fossil fuels for energy.
Fuel cells are an attractive alternative to oil dependence. Instead of polluting, fuel cells produce clean water as a by-product. While engineers have temporarily focused on producing hydrogen from various fossil sources such as gasoline or natural gas, renewable, environmentally friendly ways to produce hydrogen in the future are being explored. The most promising, naturally, will be the process of producing hydrogen from water
Dependence on oil and global warming- an international problem. Several countries are jointly involved in promoting research and development for fuel cell technology.
It is clear that scientists and manufacturers have a lot of work to do before fuel cells become an alternative to modern methods of energy production. Yet, with worldwide support and global cooperation, a viable fuel cell power system could become a reality within just a couple of decades.
A fuel cell is an electrochemical energy conversion device that converts hydrogen and oxygen into electricity through a chemical reaction. As a result of this process, water is formed and a large amount of heat is released. A fuel cell is very similar to a battery that can be charged and then use the stored electrical energy.
William R. Grove is considered the inventor of the fuel cell, who invented it back in 1839. In this fuel cell, a solution of sulfuric acid was used as an electrolyte, and hydrogen was used as a fuel, which was combined with oxygen in an oxidizing agent. It should be noted that until recently, fuel cells were used only in laboratories and on spacecraft.
In the future, fuel cells will be able to compete with many other energy conversion systems (including gas turbines in power plants), internal combustion engines in cars and electric batteries in portable devices. Internal combustion engines burn fuel and use the pressure created by the expansion of combustion gases to perform mechanical work. Batteries store electrical energy, then convert it into chemical energy, which can be converted back into electrical energy if necessary. Fuel cells are potentially very efficient. Back in 1824, the French scientist Carnot proved that the compression-expansion cycles of an internal combustion engine cannot provide an efficiency of conversion of thermal energy (which is the chemical energy of burning fuel) into mechanical energy above 50%. A fuel cell has no moving parts (at least not within the cell itself) and therefore they do not obey Carnot's law. Naturally, they will have greater than 50% efficiency and are especially effective at low loads. Thus, fuel cell vehicles are poised to become (and have already proven to be) more fuel efficient than conventional vehicles in real-world driving conditions.
The fuel cell produces a constant voltage electric current that can be used to drive the electric motor, lighting, and other electrical systems in the vehicle. There are several types of fuel cells, differing in the chemical processes used. Fuel cells are usually classified by the type of electrolyte they use. Some types of fuel cells are promising for power plant propulsion, while others may be useful for small portable devices or for powering cars.
The alkaline fuel cell is one of the very first cells developed. They have been used in the US space program since the 1960s. Such fuel cells are very susceptible to contamination and therefore require very pure hydrogen and oxygen. They are also very expensive, so this type of fuel cell will likely not see widespread use in automobiles.
Fuel cells based on phosphoric acid can find application in low-power stationary installations. They operate at fairly high temperatures and therefore take a long time to warm up, which also makes them ineffective for use in cars.
Solid oxide fuel cells are better suited for large stationary power generators that could supply power to factories or communities. This type of fuel cell operates at very high temperatures (around 1000 °C). The high operating temperature creates certain problems, but on the other hand there is an advantage - the steam produced by the fuel cell can be sent to turbines to generate more electricity. Overall, this improves the overall efficiency of the system.
One of the most promising systems is the proton exchange membrane fuel cell (PEMFC - Protone Exchange Membrane Fuel Cell). Currently, this type of fuel cell is the most promising because it can power cars, buses and other vehicles.
Chemical processes in a fuel cell
Fuel cells use an electrochemical process to combine hydrogen with oxygen obtained from the air. Like batteries, fuel cells use electrodes (solid electrical conductors) located in an electrolyte (electrically conductive medium). When hydrogen molecules come into contact with the negative electrode (anode), the latter are separated into protons and electrons. Protons pass through a proton exchange membrane (POEM) to the positive electrode (cathode) of the fuel cell, producing electricity. A chemical combination of hydrogen and oxygen molecules occurs to form water as a byproduct of this reaction. The only kind emissions from the fuel cell - water vapor.
The electricity produced by fuel cells can be used in a vehicle's electric powertrain (consisting of an electrical power converter and an AC induction motor) to provide mechanical energy to propel the vehicle. The job of a power converter is to convert the direct electrical current produced by the fuel cells into alternating current, on which the vehicle's traction motor operates.

Diagram of a fuel cell with a proton exchange membrane:
1 - anode;
2 - proton exchange membrane (PEM);
3 - catalyst (red);
4 - cathode
Proton exchange membrane fuel cell (PEMFC) uses one of the simplest reactions of any fuel cell.

Single cell fuel cell
Let's look at how a fuel cell works. The anode, the negative terminal of the fuel cell, conducts electrons that are freed from hydrogen molecules so that they can be used in the external electrical circuit. To do this, channels are engraved in it, distributing hydrogen evenly over the entire surface of the catalyst. The cathode (positive pole of the fuel cell) has etched channels that distribute oxygen across the surface of the catalyst. It also conducts electrons back from the outer loop (circuit) to the catalyst, where they can combine with hydrogen ions and oxygen to form water. The electrolyte is a proton exchange membrane. This is a special material that is similar to ordinary plastic, but has the ability to allow positively charged ions to pass through and block the passage of electrons.
A catalyst is a special material that facilitates the reaction between oxygen and hydrogen. The catalyst is usually made from platinum powder applied in a very thin layer to carbon paper or cloth. The catalyst must be rough and porous so that its surface can come into maximum contact with hydrogen and oxygen. The platinum-coated side of the catalyst is in front of the proton exchange membrane (PEM).
Hydrogen gas (H2) is supplied to the fuel cell under pressure from the anode. When an H2 molecule comes into contact with platinum on the catalyst, it splits into two parts, two ions (H+) and two electrons (e–). The electrons are conducted through the anode, where they pass through an external loop (circuit) doing useful work (such as driving an electric motor) and return at the cathode side of the fuel cell.
Meanwhile, on the cathode side of the fuel cell, oxygen gas (O 2 ) is forced through the catalyst, where it forms two oxygen atoms. Each of these atoms has a strong negative charge, which attracts two H+ ions across the membrane, where they combine with an oxygen atom and two electrons from the outer circuit to form a water molecule (H 2 O).
This reaction in a single fuel cell produces approximately 0.7 W of power. To raise power to the required level, many individual fuel cells must be combined to form a fuel cell stack.
POM fuel cells operate at relatively low temperatures (around 80°C), meaning they can be quickly brought up to operating temperature and do not require expensive cooling systems. Continuous improvements in the technologies and materials used in these cells have brought their power closer to the point where a battery of such fuel cells, occupying a small part of the trunk of a car, can provide the energy needed to drive the car.
Over the past years, most of the world's leading automobile manufacturers have been investing heavily in the development of vehicle designs that use fuel cells. Many have already demonstrated fuel cell vehicles with satisfactory power and dynamics characteristics, although they were quite high cost.
The improvement of the designs of such cars is very intensive.

Fuel cell vehicle uses a power plant located under the vehicle's floor
The NECAR V is based on a Mercedes-Benz A-class car, with the entire power plant, along with fuel cells, located under the floor of the car. This constructive solution makes it possible to accommodate four passengers and luggage in the car. Here, not hydrogen, but methanol is used as fuel for the car. Methanol, using a reformer (a device that converts methanol into hydrogen), is converted into hydrogen necessary to power the fuel cell. Using a reformer on board a car makes it possible to use almost any hydrocarbons as fuel, which allows you to refuel a fuel cell car using the existing network of gas stations. In theory, fuel cells produce nothing but electricity and water. Converting fuel (gasoline or methanol) into hydrogen needed for a fuel cell somewhat reduces the environmental appeal of such a car.
Honda, which has been involved in fuel cells since 1989, produced a small batch of Honda FCX-V4 vehicles in 2003 with proton exchange membrane fuel cells from Ballard. These fuel cells generate 78 kW of electrical power, and traction electric motors with a power of 60 kW and a torque of 272 Nm are used to drive the drive wheels. A fuel cell car, compared to a traditional car, has a weight of approximately 40% less, which ensures it has excellent dynamics, and the supply of compressed hydrogen allows it to run up to 355 km.

The Honda FCX uses electric energy generated by fuel cells to drive.
The Honda FCX is the world's first fuel cell vehicle to receive government certification in the United States. The car is certified according to ZEV standards - Zero Emission Vehicle (zero pollution vehicle). Honda is not going to sell these cars yet, but is leasing about 30 cars per unit. California and Tokyo, where hydrogen refueling infrastructure already exists.

General Motors' Hy Wire concept vehicle has a fuel cell powertrain
General Motors is conducting extensive research into the development and creation of fuel cell vehicles.

Hy Wire car chassis
The GM Hy Wire concept car was issued 26 patents. The basis of the car is a functional platform 150 mm thick. Inside the platform are hydrogen tanks, a fuel cell powertrain and vehicle control systems using the latest drive-by-wire technologies. The Hy Wire vehicle's chassis is a thin platform that encloses all of the vehicle's major structural elements: hydrogen tanks, fuel cells, batteries, electric motors and control systems. This approach to design makes it possible to change car bodies during operation. The company is also testing prototype Opel fuel cell cars and designing a plant for the production of fuel cells.

Design of a "safe" liquefied hydrogen fuel tank:
1 - filling device;
2 - external tank;
3 - supports;
4 - level sensor;
5 - internal tank;
6 - filling line;
7 - insulation and vacuum;
8 - heater;
9 - mounting box
BMW pays a lot of attention to the problem of using hydrogen as a fuel for cars. Together with Magna Steyer, renowned for its work on the use of liquefied hydrogen in space exploration, BMW has developed a fuel tank for liquefied hydrogen that can be used in cars.

Tests have confirmed the safety of using a liquid hydrogen fuel tank
The company conducted a series of tests for the safety of the structure using standard methods and confirmed its reliability.
In 2002, at the motor show in Frankfurt am Main (Germany), the Mini Cooper Hydrogen, which uses liquefied hydrogen as fuel, was shown. The fuel tank of this car takes up the same space as a regular gas tank. Hydrogen in this car is not used for fuel cells, but as fuel for the internal combustion engine.

The world's first production car with a fuel cell instead of a battery
In 2003, BMW announced the production of the first production car with a fuel cell, the BMW 750 hL. A fuel cell battery is used instead of a traditional battery. This car has a 12-cylinder internal combustion engine running on hydrogen, and the fuel cell serves as an alternative to a conventional battery, allowing the air conditioner and other electrical consumers to operate when the car is parked for long periods without the engine running.

Hydrogen filling is carried out by a robot, the driver is not involved in this process
The same BMW company has also developed robotic refueling dispensers that provide fast and safe refueling of cars with liquefied hydrogen.
Appearance in last years The large number of developments aimed at creating cars using alternative fuels and alternative powertrains suggests that internal combustion engines, which have dominated automobiles for the past century, will eventually give way to cleaner, more efficient and quieter designs. Their widespread adoption is currently constrained not by technical, but rather by economic and social problems. For their widespread use, it is necessary to create a certain infrastructure for the development of the production of alternative fuels, the creation and distribution of new gas stations and to overcome a number of psychological barriers. The use of hydrogen as a vehicle fuel will require addressing issues of storage, delivery and distribution, with serious safety measures in place.
Hydrogen is theoretically available in unlimited quantities, but its production is very energy intensive. In addition, to convert cars to run on hydrogen fuel, it is necessary to make two big changes to the power system: first, switching its operation from gasoline to methanol, and then, over a period of time, to hydrogen. It will be some time before this issue is resolved.
Fuel cell ( Fuel Cell) is a device that converts chemical energy into electrical energy. It is similar in principle to a conventional battery, but differs in that its operation requires a constant supply of substances from the outside for the electrochemical reaction to occur. Hydrogen and oxygen are supplied to the fuel cells, and the output is electricity, water and heat. Their advantages include environmental friendliness, reliability, durability and ease of operation. Unlike conventional batteries, electrochemical converters can operate virtually indefinitely as long as fuel is supplied. They don't have to be charged for hours until they're fully charged. Moreover, the cells themselves can charge the battery while the car is parked with the engine turned off.
The most widely used fuel cells in hydrogen vehicles are proton membrane fuel cells (PEMFCs) and solid oxide fuel cells (SOFCs).
A proton exchange membrane fuel cell works as follows. Between the anode and cathode there is a special membrane and a platinum-coated catalyst. Hydrogen is supplied to the anode, and oxygen (for example, from air) is supplied to the cathode. At the anode, hydrogen is decomposed into protons and electrons with the help of a catalyst. Hydrogen protons pass through the membrane and reach the cathode, and electrons are transferred to the external circuit (the membrane does not allow them to pass through). The potential difference thus obtained leads to the generation of electric current. On the cathode side, hydrogen protons are oxidized by oxygen. As a result, water vapor appears, which is the main element of car exhaust gases. Possessing high efficiency, PEM cells have one significant drawback - their operation requires pure hydrogen, the storage of which is a rather serious problem.
If such a catalyst is found that replaces expensive platinum in these cells, then a cheap fuel cell for generating electricity will immediately be created, which means the world will get rid of oil dependence.
Solid Oxide Cells
Solid oxide SOFC cells are much less demanding on fuel purity. In addition, thanks to the use of a POX reformer (Partial Oxidation), such cells can consume regular gasoline as fuel. The process of converting gasoline directly into electricity is as follows. In a special device - a reformer, at a temperature of about 800 ° C, gasoline evaporates and decomposes into its constituent elements.
This releases hydrogen and carbon dioxide. Further, also under the influence of temperature and using SOFC directly (consisting of a porous ceramic material based on zirconium oxide), hydrogen is oxidized by oxygen in the air. After obtaining hydrogen from gasoline, the process continues according to the scenario described above, with only one difference: the SOFC fuel cell, unlike devices operating on hydrogen, is less sensitive to impurities in the original fuel. So the quality of gasoline should not affect the performance of the fuel cell.
The high operating temperature of SOFC (650–800 degrees) is a significant drawback; the warm-up process takes about 20 minutes. But excess heat is not a problem, since it is completely removed by the remaining air and exhaust gases produced by the reformer and the fuel cell itself. This allows the SOFC system to be integrated into a vehicle as a separate device in a thermally insulated housing.
The modular structure allows you to achieve the required voltage by connecting a set of standard cells in series. And, perhaps most importantly from the point of view of the implementation of such devices, SOFC does not contain very expensive platinum-based electrodes. It is the high cost of these elements that is one of the obstacles in the development and dissemination of PEMFC technology.
Types of fuel cells

Currently, there are the following types of fuel cells:
- A.F.C.– Alkaline Fuel Cell (alkaline fuel cell);
- PAFC– Phosphoric Acid Fuel Cell (phosphoric acid fuel cell);
- PEMFC– Proton Exchange Membrane Fuel Cell (fuel cell with a proton exchange membrane);
- DMFC– Direct Methanol Fuel Cell (fuel cell with direct breakdown of methanol);
- MCFC– Molten Carbonate Fuel Cell (fuel cell of molten carbonate);
- SOFC– Solid Oxide Fuel Cell (solid oxide fuel cell).
Fuel cells (electrochemical generators) represent a very efficient, durable, reliable and environmentally friendly method of generating energy. Initially, they were used only in the space industry, but today electrochemical generators are increasingly used in various fields: power supplies for mobile phones and laptops, vehicle engines, autonomous power sources for buildings, and stationary power plants. Some of these devices operate as laboratory prototypes, while others are used for demonstration purposes or are undergoing pre-production testing. However, many models are already used in commercial projects and are mass-produced.
Device
Fuel cells are electrochemical devices capable of providing a high conversion rate of existing chemical energy into electrical energy.
The fuel cell device includes three main parts:
- Power generation section;
- CPU;
- Voltage transformer.
The main part of the fuel cell is the power generation section, which is a battery made of individual fuel cells. A platinum catalyst is included in the structure of the fuel cell electrodes. Using these cells, a constant electric current is created.
One of these devices has the following characteristics: at a voltage of 155 volts, 1400 amperes are produced. The battery dimensions are 0.9 m in width and height, and 2.9 m in length. The electrochemical process in it is carried out at a temperature of 177 °C, which requires heating of the battery at the time of start-up, as well as heat removal during its operation. For this purpose, a separate water circuit is included in the fuel cell, and the battery is equipped with special cooling plates.
The fuel process converts natural gas into hydrogen, which is required for an electrochemical reaction. The main element of the fuel processor is the reformer. In it, natural gas (or other hydrogen-containing fuel) interacts at high pressure and high temperature (about 900 ° C) with water vapor under the action of a nickel catalyst.
To maintain the required temperature of the reformer there is a burner. The steam required for reforming is created from the condensate. An unstable direct current is generated in the fuel cell battery and a voltage converter is used to convert it.
Also in the voltage converter block there are:
- Control devices.
- Safety interlock circuits that shut down the fuel cell during various faults.
Operating principle
The simplest proton exchange membrane cell consists of a polymer membrane that is located between the anode and cathode, as well as the cathode and anode catalysts. The polymer membrane is used as an electrolyte.
- The proton exchange membrane looks like a thin solid organic compound of small thickness. This membrane works as an electrolyte; in the presence of water, it separates the substance into negatively and positively charged ions.
- Oxidation begins at the anode, and reduction occurs at the cathode. The cathode and anode in a PEM cell are made of porous material; it is a mixture of platinum and carbon particles. Platinum acts as a catalyst, which promotes the dissociation reaction. The cathode and anode are made porous so that oxygen and hydrogen pass through them freely.
- The anode and cathode are located between two metal plates, they supply oxygen and hydrogen to the cathode and anode, and remove electrical energy, heat and water.
- Through channels in the plate, hydrogen molecules enter the anode, where the molecules are decomposed into atoms.
- As a result of chemisorption under the influence of a catalyst, hydrogen atoms are converted into positively charged hydrogen ions H+, that is, protons.
- Protons diffuse to the cathode through the membrane, and a flow of electrons goes to the cathode through a special external electrical circuit. A load is connected to it, that is, a consumer of electrical energy.
- Oxygen, which is supplied to the cathode, upon exposure, enters into a chemical reaction with electrons from the external electrical circuit and hydrogen ions from the proton exchange membrane. As a result of this chemical reaction, water appears.
The chemical reaction that occurs in other types of fuel cells (for example, with an acidic electrolyte in the form of orthophosphoric acid H3PO4) is completely identical to the reaction of a device with a proton exchange membrane.
Kinds
Currently, several types of fuel cells are known, which differ in the composition of the electrolyte used:
- Fuel cells based on orthophosphoric or phosphoric acid (PAFC, Phosphoric Acid Fuel Cells).
- Devices with proton exchange membrane (PEMFC, Proton Exchange Membrane Fuel Cells).
- Solid oxide fuel cells (SOFC, Solid Oxide Fuel Cells).
- Electrochemical generators based on molten carbonate (MCFC, Molten Carbonate Fuel Cells).
Currently, electrochemical generators using PAFC technology have become more widespread.
Application
Today, fuel cells are used in the Space Shuttle, reusable spacecraft. They use 12 W units. They generate all the electricity on the spacecraft. The water that is formed during the electrochemical reaction is used for drinking, including for cooling equipment.
Electrochemical generators were also used to power the Soviet Buran, a reusable spacecraft.
Fuel cells are also used in the civilian sector.
- Stationary installations with a power of 5–250 kW and above. They are used as autonomous sources for heat and power supply to industrial, public and residential buildings, emergency and backup power supplies, and uninterruptible power supplies.
- Portable units with a power of 1–50 kW. They are used for space satellites and ships. Instances are created for golf carts, wheelchairs, railway and freight refrigerators, and road signs.
- Mobile installations with a power of 25–150 kW. They are beginning to be used in military ships and submarines, including cars and other vehicles. Prototypes have already been created by such automotive giants as Renault, Neoplan, Toyota, Volkswagen, Hyundai, Nissan, VAZ, General Motors, Honda, Ford and others.
- Microdevices with a power of 1–500 W. They find application in advanced handheld computers, laptops, consumer electronic devices, mobile phones, and modern military devices.
Peculiarities
- Some of the energy from the chemical reaction in each fuel cell is released as heat. Refrigeration required. In an external circuit, the flow of electrons creates a direct current that is used to do work. Stopping the movement of hydrogen ions or opening the external circuit leads to the stop of the chemical reaction.
- The amount of electricity that fuel cells create is determined by gas pressure, temperature, geometric dimensions, and type of fuel cell. To increase the amount of electricity produced by the reaction, fuel cells can be made larger, but in practice several cells are used, which are combined into batteries.
- The chemical process in some types of fuel cells can be reversed. That is, when a potential difference is applied to the electrodes, water can be decomposed into oxygen and hydrogen, which will be collected on the porous electrodes. When the load is turned on, such a fuel cell will generate electrical energy.
Prospects
Currently, electrochemical generators require large initial costs to be used as the main source of energy. With the introduction of more stable membranes with high conductivity, efficient and cheap catalysts, and alternative sources of hydrogen, fuel cells will become highly economically attractive and will be implemented everywhere.
- Cars will run on fuel cells; there will be no internal combustion engines at all. Water or solid-state hydrogen will be used as an energy source. Refueling will be simple and safe, and driving will be environmentally friendly - only water vapor will be produced.
- All buildings will have their own portable fuel cell power generators.
- Electrochemical generators will replace all batteries and will be installed in any electronics and household appliances.
Advantages and disadvantages
Each type of fuel cell has its own disadvantages and advantages. Some require high quality fuel, others have complex design, need high operating temperature.
In general, the following advantages of fuel cells can be noted:
- environmental safety;
- electrochemical generators do not need to be recharged;
- electrochemical generators can create energy constantly, they do not care about external conditions;
- flexibility in scale and portability.
Among the disadvantages are:
- technical difficulties with fuel storage and transportation;
- imperfect elements of the device: catalysts, membranes, and so on.
I insert the filler hose fitting into the fuel filler neck and turn it half a turn to seal the connection. A click of the toggle switch - and the blinking LED on the gas pump with a huge inscription h3 indicates that refueling has started. A minute - and the tank is full, you can go!
Elegant body contours, ultra-low suspension, low-profile slicks give off a real racing breed. Through the transparent cover, an intricate network of pipelines and cables is visible. I've already seen a similar solution somewhere... Oh yes, on the Audi R8 the engine is also visible through the rear window. But on Audi it is traditional gasoline, and this car runs on hydrogen. Like the BMW Hydrogen 7, but unlike the latter, there is no internal combustion engine. The only moving parts are the steering gear and the electric motor rotor. And the energy for it is provided by a fuel cell. This car was produced by the Singaporean company Horizon Fuel Cell Technologies, specializing in the development and production of fuel cells. In 2009, the British company Riversimple already introduced an urban hydrogen car powered by Horizon Fuel Cell Technologies fuel cells. It was developed in collaboration with the Universities of Oxford and Cranfield. But Horizon H-racer 2.0 is a solo development.
The fuel cell consists of two porous electrodes coated with a layer of catalyst and separated by a proton exchange membrane. Hydrogen at the anode catalyst is converted into protons and electrons, which travel through the anode and an external electrical circuit to the cathode, where hydrogen and oxygen recombine to form water.
"Go!" - in Gagarin style he nudges me with his elbow Chief Editor. But not so fast: first you need to “warm up” the fuel cell at part load. I switch the toggle switch to “warm up” mode and wait for the allotted time. Then, just in case, I top up the tank until it’s full. Now let's go: the car, the engine humming smoothly, moves forward. The dynamics are impressive, although, by the way, what else can you expect from an electric car - the torque is constant at any speed. Although not for long - a full tank of hydrogen lasts only a few minutes (Horizon promises to release a new version in the near future, in which hydrogen is not stored as a gas under pressure, but is retained by a porous material in the adsorber). And, frankly speaking, it is not very controlled - there are only two buttons on the remote control. But in any case, it’s a pity that this is only a radio-controlled toy, which cost us $150. We wouldn't mind taking a ride in a real fuel cell car. power plant.
The tank, an elastic rubber container inside a rigid casing, stretches when refueling and works as a fuel pump, “squeezing” hydrogen into the fuel cell. In order not to “overfill” the tank, one of the fittings is connected with a plastic tube to the emergency pressure relief valve.

Gas station
Do it yourself
The Horizon H-racer 2.0 machine is supplied as a kit for large-scale assembly (do-it-yourself type), you can buy it, for example, on Amazon. However, assembling it is not difficult - just put the fuel cell in place and secure it with screws, connect the hoses to the hydrogen tank, fuel cell, filler neck and emergency valve, and all that remains is to put the upper part of the body in place, not forgetting the front and rear bumpers. The kit includes a filling station that produces hydrogen by electrolysis of water. It is powered by two AA batteries, and if you want the energy to be completely “clean”, by solar panels (they are also included in the kit).
www.popmech.ru
How to make a fuel cell with your own hands?
Of course, the simplest solution to the problem of ensuring the constant operation of fuel-free systems is to purchase a ready-made secondary energy source on a hydraulic or any other basis, but in this case it will certainly not be possible to avoid additional costs, and in this process it is quite difficult to consider any idea for flight of creative thought. In addition, making a fuel cell with your own hands is not at all as difficult as you might think at first glance, and even the most inexperienced craftsman can cope with the task if desired. In addition, a more than pleasant bonus will be the low cost of creating this element, because despite all its benefits and importance, you can absolutely easily make do with the means you already have at hand.
In this case, the only nuance that must be taken into account before completing the task is that you can make an extremely low-power device with your own hands, and the implementation of more advanced and complex installations should still be left to qualified specialists. As for the order of work and the sequence of actions, the first step is to complete the body, for which it is best to use thick-walled plexiglass (at least 5 centimeters). For gluing the walls of the case and installing internal partitions, for which it is best to use thinner plexiglass (3 millimeters is enough), ideally use two-composite glue, although if you really want, you can do high-quality soldering yourself, using the following proportions: per 100 grams of chloroform - 6 grams shavings from the same plexiglass.
In this case, the process must be carried out exclusively under a hood. In order to equip the case with the so-called drain system, it is necessary to carefully drill a through hole in its front wall, the diameter of which will exactly match the dimensions of the rubber plug, which serves as a kind of gasket between the case and the glass drain tube. As for the size of the tube itself, ideally its width should be five to six millimeters, although it all depends on the type of structure being designed. It is more likely to say that the old gas mask listed in the list of necessary elements for making a fuel cell will cause some surprise among potential readers of this article. Meanwhile, the entire benefit of this device lies in the activated carbon located in the compartments of its respirator, which can later be used as electrodes.
Since we are talking about a powdery consistency, to improve the design you will need nylon stockings, from which you can easily make a bag and put the coal in it, otherwise it will simply spill out of the hole. As for the distribution function, the concentration of fuel occurs in the first chamber, while the oxygen necessary for the normal functioning of the fuel cell, on the contrary, will circulate in the last, fifth compartment. The electrolyte itself, located between the electrodes, should be soaked in a special solution (gasoline with paraffin in a ratio of 125 to 2 milliliters), and this must be done before placing the air electrolyte in the fourth compartment. To ensure proper conductivity, copper plates with pre-soldered wires are laid on top of the coal, through which electricity will be transmitted from the electrodes.
This design stage can be safely considered the final stage, after which the finished device is charged, for which an electrolyte will be needed. To prepare it, you need to mix in equal parts ethyl alcohol with distilled water and begin gradually introducing caustic potassium at the rate of 70 grams per glass of liquid. Carrying out the first test of the manufactured device consists of simultaneously filling the first (fuel liquid) and third (electrolyte made of ethyl alcohol and caustic potassium) containers of the plexiglass housing.
uznay-kak.ru
Hydrogen fuel cells | LAVENT
I have long wanted to tell you about another direction of the Alfaintek company. This is the development, sale and service of hydrogen fuel cells. I would like to immediately explain the situation with these fuel cells in Russia.
Due to the fairly high cost and the complete lack of hydrogen stations for charging these fuel cells, their sale in Russia is not expected. Nevertheless, in Europe, especially in Finland, these fuel cells are gaining popularity every year. What's the secret? Let's get a look.  This device is environmentally friendly, easy to use and effective. It comes to the aid of a person where he needs electrical energy. You can take it with you on the road, on a hike, or use it in your country house or apartment as an autonomous source of electricity.
This device is environmentally friendly, easy to use and effective. It comes to the aid of a person where he needs electrical energy. You can take it with you on the road, on a hike, or use it in your country house or apartment as an autonomous source of electricity.
Electricity in a fuel cell is generated by a chemical reaction of hydrogen from the tank with metal hydride and oxygen from the air. The cylinder is not explosive and can be stored in your closet for years, waiting in the wings. This is perhaps one of the main advantages of this hydrogen storage technology. It is the storage of hydrogen that is one of the main problems in the development of hydrogen fuel. Unique new lightweight fuel cells that convert hydrogen into conventional electricity safely, quietly and emission-free.
This type of electricity can be used in places where there is no central electricity, or as an emergency power source.
Unlike conventional batteries, which need to be charged and disconnected from the electrical consumer during the charging process, a fuel cell works as a “smart” device. This technology provides uninterrupted power throughout the entire period of use thanks to the unique power saving function when changing the fuel container, which allows the user to never turn off the consumer. In a closed case, fuel cells can be stored for several years without losing the volume of hydrogen and reducing their power.
The fuel cell is intended for scientists and researchers, law enforcement agencies, rescue workers, ship and marina owners, and anyone else who needs reliable source food in case of emergency.  You can get 12 volts or 220 volts and then you will have enough energy to run your TV, stereo, refrigerator, coffee maker, kettle, vacuum cleaner, drill, microstove and other electrical appliances.
You can get 12 volts or 220 volts and then you will have enough energy to run your TV, stereo, refrigerator, coffee maker, kettle, vacuum cleaner, drill, microstove and other electrical appliances.
Hydrocell fuel cells can be sold as a single unit or in batteries of 2-4 cells. Two or four elements can be combined to either increase power or increase amperage. 

OPERATING TIME OF HOUSEHOLD APPLIANCES WITH FUEL CELLS
| Electrical appliances | Operating time per day (min.) | Required power per day (Wh) | Operating time with fuel cells |
|||
| Electric kettle | ||||||
| Coffee maker | ||||||
| Microslab | ||||||
| TV | ||||||
| 1 light bulb 60W | ||||||
| 1 light bulb 75W | ||||||
| 3 bulbs 60W | ||||||
| Computer laptop | ||||||
| Fridge | ||||||
| Energy saving lamp | ||||||
* - continuous operation
Fuel cells are fully charged at special hydrogen stations. But what if you travel far from them and there is no way to recharge? Especially for such cases, Alfaintek specialists have developed cylinders for storing hydrogen, with which fuel cells will work much longer.
Two types of cylinders are available: NS-MN200 and NS-MN1200. The assembled NS-MN200 is slightly larger than a Coca-Cola can, it holds 230 liters of hydrogen, which corresponds to 40Ah (12V), and weighs only 2.5 kg .The NS-MN1200 metal hydride cylinder holds 1200 liters of hydrogen, which corresponds to 220Ah (12V). The weight of the cylinder is 11 kg.
The metal hydride technique is a safe and easy way to store, transport and use hydrogen. When stored as a metal hydride, hydrogen is in the form chemical compound, and not in gaseous form. This method makes it possible to obtain a sufficiently high energy density. The advantage of using metal hydride is that the pressure inside the cylinder is only 2-4 bar. The cylinder is not explosive and can be stored for years without reducing the volume of the substance. Since the hydrogen is stored as a metal hydride, the purity of the hydrogen obtained from the cylinder is very high at 99.999%. Metal hydride hydrogen storage cylinders can be used not only with HC 100,200,400 fuel cells, but also in other cases where pure hydrogen is needed. The cylinders can be easily connected to a fuel cell or other device using a quick-connect connector and flexible hose.
It is a pity that these fuel cells are not sold in Russia. But among our population there are so many people who need them. Well, we'll wait and see, and you'll see, we'll have some. In the meantime, we will buy energy-saving light bulbs imposed by the state.
P.S. It looks like the topic has finally faded into oblivion. So many years after this article was written, nothing has come of it. Maybe I’m not looking everywhere, of course, but what catches my eye is not at all pleasing. The technology and idea are good, but they haven’t found any development yet.
lavent.ru
The fuel cell is a future that starts today!
The beginning of the 21st century considers ecology as one of the most important global challenges. And the first thing that should be paid attention to in the current conditions is the search and use of alternative energy sources. They are the ones who are able to prevent pollution of our environment, as well as completely abandon the continuously rising prices of hydrocarbon-based fuels.
 Already today, energy sources such as solar cells and wind turbines have found application. But, unfortunately, their disadvantage is associated with dependence on the weather, as well as on the season and time of day. For this reason, their use in astronautics, aircraft and automotive industries is gradually being abandoned, and for stationary use they are equipped with secondary power sources - batteries.
Already today, energy sources such as solar cells and wind turbines have found application. But, unfortunately, their disadvantage is associated with dependence on the weather, as well as on the season and time of day. For this reason, their use in astronautics, aircraft and automotive industries is gradually being abandoned, and for stationary use they are equipped with secondary power sources - batteries.
However, the best solution is a fuel cell, since it does not require constant energy recharging. This is a device that is capable of processing and converting various types of fuel (gasoline, alcohol, hydrogen, etc.) directly into electrical energy.
A fuel cell works on the following principle: fuel is supplied from the outside, which is oxidized by oxygen, and the energy released is converted into electricity. This principle of operation ensures almost eternal operation.
 Since the end of the 19th century, scientists have studied the fuel cell itself and constantly developed new modifications of it. So, today, depending on operating conditions, there are alkaline or alkaline (AFC), direct borohydrate (DBFC), electro-galvanic (EGFC), direct methanol (DMFC), zinc-air (ZAFC), microbial (MFC), models on formic acid(DFAFC) and metal hydrides (MHFC).
Since the end of the 19th century, scientists have studied the fuel cell itself and constantly developed new modifications of it. So, today, depending on operating conditions, there are alkaline or alkaline (AFC), direct borohydrate (DBFC), electro-galvanic (EGFC), direct methanol (DMFC), zinc-air (ZAFC), microbial (MFC), models on formic acid(DFAFC) and metal hydrides (MHFC).
One of the most promising is the hydrogen fuel cell. The use of hydrogen in power plants is accompanied by a significant release of energy, and the exhaust from such a device is pure water vapor or drinking water, which do not pose any threat to the environment.
 The successful testing of fuel cells of this type on spacecraft has recently aroused considerable interest among manufacturers of electronics and various equipment. Thus, the PolyFuel company presented a miniature hydrogen fuel cell for laptops. But the too high cost of such a device and the difficulties in unhindered refueling limit its industrial production and wide distribution. Honda has also been producing automotive fuel cells for over 10 years. However, this type of transport does not go on sale, but only for the official use of company employees. The cars are under the supervision of engineers.
The successful testing of fuel cells of this type on spacecraft has recently aroused considerable interest among manufacturers of electronics and various equipment. Thus, the PolyFuel company presented a miniature hydrogen fuel cell for laptops. But the too high cost of such a device and the difficulties in unhindered refueling limit its industrial production and wide distribution. Honda has also been producing automotive fuel cells for over 10 years. However, this type of transport does not go on sale, but only for the official use of company employees. The cars are under the supervision of engineers.
Many people wonder whether it is possible to assemble a fuel cell with their own hands. After all, a significant advantage of a homemade device will be a minor investment, in contrast to an industrial model. For the miniature model, you will need 30 cm of platinum-coated nickel wire, a small piece of plastic or wood, a 9-volt battery clip and the battery itself, clear adhesive tape, a glass of water and a voltmeter. Such a device will allow you to see and understand the essence of the work, but, of course, it will not be possible to generate electricity for the car.
fb.ru
Hydrogen fuel cells: a little history | Hydrogen
In our time, the problem of the shortage of traditional energy resources and the deterioration of the planet’s ecology as a whole due to their use is particularly acute. That is why, recently, significant financial resources and intellectual resources have been spent on the development of potentially promising substitutes for hydrocarbon fuels. Hydrogen may become such a substitute in the very near future, since its use in power plants is accompanied by the release of a large amount of energy, and the exhaust is water vapor, that is, it does not pose a danger to the environment.

Despite some technical difficulties that still exist in the implementation of hydrogen-based fuel cells, many car manufacturers have appreciated the promise of the technology and are already actively developing prototypes of production cars capable of using hydrogen as the main fuel. Back in two thousand and eleven, Daimler AG presented conceptual Mercedes-Benz models with hydrogen power plants. In addition, the Korean company Hyndayi has officially announced that it no longer intends to develop electric cars, but will concentrate all its efforts on developing an affordable hydrogen car.
Despite the fact that the very idea of using hydrogen as a fuel is not wild for many, most have no idea how fuel cells that use hydrogen work and what is so remarkable about them.
To understand the importance of the technology, we suggest looking at the history of hydrogen fuel cells.
The first person to describe the potential of using hydrogen in a fuel cell was a German by nationality, Christian Friedrich. Back in 1838, he published his work in a famous scientific journal of the time.
The very next year, a prototype of a workable hydrogen battery was created by a judge from Uhls, Sir William Robert Grove. However, the power of the device was too small even by the standards of that time, so its practical use was out of the question.
As for the term “fuel cell,” it owes its existence to scientists Ludwig Mond and Charles Langer, who in 1889 attempted to create a fuel cell operating on air and coke oven gas. According to other sources, the term was first used by William White Jaques, who first decided to use phosphoric acid in an electrolyte.
In the 1920s, a number of studies were carried out in Germany, which resulted in the discovery of solid oxide fuel cells and ways to use the carbonate cycle. It is noteworthy that these technologies are effectively used in our time.
In 1932, engineer Francis T Bacon began work on directly researching hydrogen-based fuel cells. Before him, scientists used an established scheme - porous platinum electrodes were placed in sulfuric acid. The obvious disadvantage of such a scheme lies, first of all, in its unjustified high cost due to the use of platinum. In addition, the use of caustic sulfuric acid posed a threat to the health, and sometimes even the life, of researchers. Bacon decided to optimize the circuit and replaced platinum with nickel, and used an alkaline composition as the electrolyte.
Thanks to productive work to improve his technology, Bacon already in 1959 presented to the general public his original hydrogen fuel cell, which produced 5 kW and could power a welding machine. He called the presented device “Bacon Cell”.
In October of the same year, a unique tractor was created that ran on hydrogen and produced twenty horsepower.
In the sixties of the twentieth century, the American company General Electric developed the scheme developed by Bacon and applied it to the Apollo and NASA Gemini space programs. Experts from NASA came to the conclusion that using a nuclear reactor is too expensive, technically difficult and unsafe. In addition, we had to abandon the use of batteries together with solar panels due to their large dimensions. The solution to the problem was hydrogen fuel cells, which are capable of supplying the spacecraft with energy and its crew with clean water.

The first bus using hydrogen as fuel was built back in 1993. And prototypes of passenger cars powered by hydrogen fuel cells were presented already in 1997 by such global automobile brands as Toyota and Daimler Benz.
It’s a little strange that a promising environmentally friendly fuel, sold fifteen years ago in a car, has not yet become widespread. There are many reasons for this, the main ones, perhaps, are political and the demands for creating the appropriate infrastructure. Let's hope that hydrogen will still have its say and become a significant competitor to electric cars.(odnaknopka)
energycraft.org
Created 07/14/2012 20:44 Author: Alexey NorkinWithout energy, our material society cannot not only develop, but even exist at all. Where does the energy come from? Until recently, people used only one way to obtain it; we fought with nature, burning the extracted trophies in the furnaces of first home hearths, then steam locomotives and powerful thermal power plants.
There are no labels on the kilowatt-hours consumed by the modern average person that would indicate how many years nature worked so that civilized man could enjoy the benefits of technology, and how many years she still has to work to smooth out the damage caused to her by such a civilization. However, there is a growing understanding in society that sooner or later the illusory idyll will end. Increasingly, people are inventing ways to provide energy for their needs with minimal damage to nature.
Hydrogen fuel cells are the Holy Grail of clean energy. They process hydrogen, one of the common elements periodic table and release only water, the most abundant substance on the planet. The rosy picture is spoiled by people's lack of access to hydrogen as a substance. There is a lot of it, but only in a bound state, and extracting it is much more difficult than pumping oil out of the depths or digging up coal.
One of the options for clean and environmentally friendly production of hydrogen is microbial fuel cells (MTB), which use microorganisms to decompose water into oxygen and hydrogen. Not everything is smooth here either. Microbes do an excellent job of producing clean fuel, but to achieve the efficiency required in practice, MTB needs a catalyst that accelerates one of the chemical reactions of the process.
This catalyst is the precious metal platinum, the cost of which makes the use of MTB economically unjustified and practically impossible.
Scientists from the University of Wisconsin-Milwaukee have found a replacement for the expensive catalyst. Instead of platinum, they proposed using cheap nanorods made from a combination of carbon, nitrogen and iron. The new catalyst consists of graphite rods with nitrogen embedded in the surface layer and iron carbide cores. During three months of testing the new product, the catalyst demonstrated capabilities higher than those of platinum. The operation of nanorods turned out to be more stable and controllable.
And most importantly, the brainchild of university scientists is much cheaper. Thus, the cost of platinum catalysts is approximately 60% of the cost of MTB, while the cost of nanorods is within 5% of their current price.
According to the creator of catalytic nanorods, Professor Junhong Chen: “Fuel cells are capable of directly converting fuel into electricity. Together, electrical energy from renewable sources can be delivered where it is needed in a clean, efficient and sustainable manner.”
Professor Chen and his team of researchers are now studying the exact characteristics of the catalyst. Their goal is to give their invention a practical focus, to make it suitable for mass production and use.
Based on materials from Gizmag
www.facepla.net
Hydrogen fuel cells and energy systems
A water-powered car may soon become a reality and hydrogen fuel cells will be installed in many homes...
Hydrogen fuel cell technology is not new. It began in 1776, when Henry Cavendish first discovered hydrogen while dissolving metals in dilute acids. The first hydrogen fuel cell was invented already in 1839 by William Grove. Since then, hydrogen fuel cells have been gradually improved and are now installed in space shuttles, supplying them with energy and serving as a source of water. Today, hydrogen fuel cell technology is on the verge of reaching the mass market, in cars, homes and portable devices.
In a hydrogen fuel cell, chemical energy (in the form of hydrogen and oxygen) is converted directly (without combustion) into electrical energy. A fuel cell consists of a cathode, electrodes and an anode. Hydrogen is fed to the anode, where it is separated into protons and electrons. Protons and electrons have different routes to the cathode. Protons move through the electrode to the cathode, and electrons pass around the fuel cells to get to the cathode. This movement creates subsequently usable electrical energy. On the other side, hydrogen protons and electrons combine with oxygen to form water.
Electrolyzers are one way to extract hydrogen from water. The process is basically the opposite of what happens with a hydrogen fuel cell. The electrolyzer consists of an anode, an electrochemical cell and a cathode. Water and voltage are applied to the anode, which splits the water into hydrogen and oxygen. Hydrogen passes through the electrochemical cell to the cathode and oxygen is supplied directly to the cathode. From there, hydrogen and oxygen can be extracted and stored. During times when electricity is not required to be generated, the accumulated gas can be removed from the storage facility and passed back through the fuel cell.
This system uses hydrogen as fuel, which is probably why there are many myths about its safety. After the explosion of the Hindenburg, many people far from science and even some scientists began to believe that the use of hydrogen is very dangerous. However, recent research has shown that the cause of this tragedy was related to the type of material that was used in the construction, and not to the hydrogen that was pumped inside. After testing the safety of hydrogen storage, it was found that storing hydrogen in fuel cells is safer than storing gasoline in a car fuel tank.
How much do modern hydrogen fuel cells cost? Companies currently offer hydrogen fuel systems that produce power for about $3,000 per kilowatt. Marketing research has established that when the cost drops to $1,500 per kilowatt, consumers in the mass energy market will be ready to switch to this type of fuel.
Hydrogen fuel cell vehicles are still more expensive than internal combustion engine vehicles, but manufacturers are exploring ways to bring the price to comparable levels. In some remote areas where there are no power lines, using hydrogen as a fuel or powering the home independently may be more economical right now than, for example, building infrastructure for traditional energy sources.
Why are hydrogen fuel cells still not widely used? At the moment, their high cost is the main problem for the spread of hydrogen fuel cells. Hydrogen fuel systems simply do not have mass demand at the moment. However, science does not stand still and in the near future a car running on water may become a real reality.
www.tesla-tehnika.biz
In the light latest events, associated with overheating, fires and even explosions of laptops due to lithium-ion batteries, one cannot help but recall new alternative technologies, which, according to most experts, in the future will be able to supplement or replace today's traditional batteries. We are talking about new power sources – fuel cells.
According to an empirical law formulated 40 years ago by one of the founders of Intel, Gordon Moore, processor performance doubles every 18 months. Batteries can't keep up with chips. Their capacity, according to experts, increases only by 10% per year.
The fuel cell operates on the basis of a cellular (porous) membrane that separates the anode and cathode spaces of the fuel cell. This membrane is coated on both sides with appropriate catalysts. Fuel is supplied to the anode; in this case, a methanol solution (methyl alcohol) is used. As a result of the chemical reaction of fuel decomposition, free charges are formed that penetrate through the membrane to the cathode. The electrical circuit is thus closed, and an electric current is created in it to power the device. This type of fuel cell is called Direct Methanol Fuel Cell (DMFC). The development of fuel cells began a long time ago, but the first results, which gave rise to talk about real competition with lithium-ion batteries, were obtained only in the last two years.
In 2004, there were about 35 manufacturers on the market for such devices, but only a few companies were able to declare significant success in this area. In January, Fujitsu presented its development - the battery had a thickness of 15 mm and contained 300 mg of a 30 percent methanol solution. A power of 15 W allowed it to power the laptop for 8 hours. A month later, a small company, PolyFuel, was the first to announce the launch of commercial production of the very membranes that should be equipped with fuel power supplies. And already in March, Toshiba demonstrated a prototype of a mobile PC running on fuel. The manufacturer stated that such a laptop can last five times longer than a laptop using a traditional battery.
In 2005, LG Chem announced the creation of its own fuel cell. About 5 years and 5 billion dollars were spent on its development. As a result, it was possible to create a device with a power of 25 W and a weight of 1 kg, connected to a laptop via a USB interface and ensuring its operation for 10 hours. This year, 2006, was also marked by a number of interesting developments. In particular, American developers from the company Ultracell demonstrated a fuel cell that provides a power of 25 W and is equipped with three replaceable cartridges with 67 percent methanol. It is capable of powering a laptop for 24 hours. The weight of the battery was about a kilogram, each cartridge weighed about 260 grams.
In addition to being able to provide greater capacity than lithium ion batteries, methanol batteries are non-explosive. The disadvantages include their rather high cost and the need to periodically change methanol cartridges.
Even if fuel batteries do not replace traditional ones, they will most likely be used in conjunction with them. According to experts, the fuel cell market in 2006 will be about $600 million, which is a fairly modest figure. However, by 2010, experts predict its threefold increase - up to 1.9 billion dollars.
Discussion of the article “Alcohol batteries are replacing lithium ones”
zemoneng
Holy shit, I found information about this device in a women's magazine.
Well, I’ll say a few words about this:
1: the inconvenience is that after 6-10 hours of operation, you will have to look for a new cartridge, which is expensive. Why should I spend money on this nonsense?
2: as far as I understand, after receiving energy from methyl alcohol, water should be released. A laptop and water are incompatible things.
3: why do you write in women's magazines? Judging by the comments “I don’t know anything.” and “What is this?”, this article is not at the level of a site dedicated to BEAUTIES.
Hydrogen fuel cells convert the chemical energy of fuel into electricity, bypassing the ineffective processes of combustion and conversion of thermal energy into mechanical energy, which involve large losses. A hydrogen fuel cell is electrochemical The device directly generates electricity as a result of highly efficient “cold” combustion of fuel. The hydrogen-air proton exchange membrane fuel cell (PEMFC) is one of the most promising fuel cell technologies.
Eight years ago in Western Europe six liquid diesel pumps were opened; they must be two hundred before the end. We are a far cry from the thousands of fast charging terminals that are hatching all over the place to encourage the spread of electric propulsion. And that's where the rub hurts. And we better announce graphene.
The batteries haven't had their last word
There's more to it than autonomy, which is why limiting charging times is slowing EV adoption. However, he recalled in a note this month to his customers that batteries have a limitation, limited to this type of probe at very high voltages. Thomas Brachman will be told that a hydrogen distribution network still needs to be built. The argument is that he sweeps his hand, recalling that the multiplication of fast charge terminals is also very expensive, due to the high cross-section of high-voltage copper cables. “It is easier and cheaper to transport liquefied hydrogen by truck from buried tanks near production sites.”

A proton-conducting polymer membrane separates two electrodes—anode and cathode. Each electrode is a carbon plate (matrix) coated with a catalyst. At the anode catalyst, molecular hydrogen dissociates and gives up electrons. Hydrogen cations are conducted through the membrane to the cathode, but electrons are given into the external circuit, since the membrane does not allow electrons to pass through.
Hydrogen is not yet a pure vector of electricity
As for the cost of the battery itself, which is a very sensitive information, Thomas Brachmann has no doubt that it can be significantly reduced as efficiency increases. “Platinum is the element that costs more.” Unfortunately, almost all hydrogen comes from fossil energy sources. Moreover, dihydrogen is just a vector of energy, and not a source from which a non-negligible part is consumed during its production, its liquefaction, and then its conversion into electricity.

At the cathode catalyst, an oxygen molecule combines with an electron (which is supplied from the electrical circuit) and an incoming proton and forms water, which is the only product of the reaction (in the form of vapor and/or liquid).
Membrane-electrode units, which are the key generating element of the energy system, are made from hydrogen fuel cells.
The car of the future behaves like a real one
The battery balance is approximately three times higher, despite losses due to heat in the drivers. Alas, the miracle car will not hit our roads except as part of public demonstrations. Brachmann, who reminds us that the natural silence of an electric car enhances the impression of living in a noisy world. Despite all the difficulties, the steering and brake pedal provide a natural consistency.
Miniature battery but improved performance
The gadget is visible, the central screen diffuses the images of the camera placed in the right mirror as soon as the turn signal is activated. Most of our American customers no longer require, and this allows us to keep prices down - justifies the chief engineer, who offers a lower tariff than. It's actually worth talking about a fuel cell stack since there are 358 that work together. The main reservoir, with a capacity of 117 liters, is pressed against the rear wall of the bench, preventing it from being folded, and the second - 24 liters, is hidden under the seat.
Advantages of hydrogen fuel cells compared to traditional solutions:
- increased specific energy intensity (500 ÷ 1000 Wh/kg),
- extended operating temperature range (-40 0 C / +40 0 C),
- absence of heat spot, noise and vibration,
- reliability at cold start,
- practically unlimited energy storage period (no self-discharge),
First two-stroke fuel cell
Despite its compact size, this new fuel cell converts dihydrogen into electrical current faster and better than its predecessor. It delivers oxygen to the pile elements at a rate previously considered incompatible with their durability. Excess water that previously limited the flow rate is best evacuated. As a result, the power per element increases by half, and efficiency reaches 60%.
This is due to the presence of a 1.7 kWh lithium-ion battery - located under the front seats, which allows additional current to be delivered under strong accelerations. Or the forecast autonomy is 460 km, ideally consistent with what the manufacturer claims.
- the ability to change the energy intensity of the system by changing the number of fuel cartridges, which provides almost unlimited autonomy,
The ability to provide almost any reasonable energy intensity of the system by changing the hydrogen storage capacity,
- high energy intensity,
- tolerance to impurities in hydrogen,
But a thousand parts facilitate air flow and optimize cooling. Even more than its predecessor, this electric car shows that the fuel cell is front and center. A big challenge for the industry and our leaders. Meanwhile, it is very smart who will know which fuel cell or battery will prevail.
A fuel cell is an electrochemical energy conversion device that can produce electricity in the form of direct current by combining a fuel and an oxidizer in a chemical reaction to produce a waste product, typically a fuel oxide.
- long service life,
- environmental friendliness and quiet operation.
Power supply systems based on hydrogen fuel cells for UAVs:
Installation of fuel cells on unmanned vehicles instead of traditional batteries, it multiplies flight duration, payload weight, and improves reliability aircraft, expand the temperature range of UAV launch and operation, lowering the limit to -40 0C. Compared to internal combustion engines, fuel cell-based systems are silent, vibration-free, and operate at low temperatures, are difficult to detect during flight, do not produce harmful emissions and allow you to effectively perform tasks from video surveillance to delivery of payloads.
Each fuel cell has two electrodes, one positive and the other negative, and the reaction that produces electricity occurs at the electrodes in the presence of an electrolyte, which carries charged particles from electrode to electrode, while electrons circulate in external wires located between the electrodes to create electricity.
The fuel cell can generate electricity continuously as long as the required flow of fuel and oxidizer is maintained. Some fuel cells produce only a few watts, while others can produce several hundred kilowatts, while smaller batteries are likely to be found in laptops and cell phones, but fuel cells are too expensive to become small generators used to produce electricity for homes and businesses.




Composition of the power supply system for UAVs:
Economic Dimensions of Fuel Cells
Using hydrogen as a fuel source entails significant costs. For this reason, hydrogen is now an uneconomic source, particularly because other less expensive sources can be used. Hydrogen production costs can vary as they reflect the cost of the resources from which it is extracted.
Battery fuel sources
Fuel cells are generally classified into the following categories: hydrogen fuel cells, organic fuel cells, metallic fuel cells, and redox batteries. When hydrogen is used as a fuel source, chemical energy is converted into electricity during the reverse hydrolysis process to produce only water and heat as waste. A hydrogen fuel cell is very low, but can be more or less high in hydrogen production, especially if produced from fossil fuels.
- - fuel cell battery,
- - Li-Po buffer battery to cover short-term peak loads,
- - electronic control system ,
- - fuel system consisting of a cylinder with compressed hydrogen or a solid source of hydrogen.
The fuel system uses high-strength lightweight cylinders and reducers to ensure maximum supply of compressed hydrogen on board. It is allowed to use different sizes of cylinders (from 0.5 to 25 liters) with reducers that provide the required hydrogen consumption.
Hydrogen batteries are divided into two categories: low temperature batteries and high temperature batteries, where high temperature batteries can also use fossil fuels directly. The latter consist of hydrocarbons such as oil or gasoline, alcohol or biomass.
Other fuel sources in batteries include, but are not limited to, alcohols, zinc, aluminum, magnesium, ionic solutions and many hydrocarbons. Other oxidizing agents include, but are not limited to, air, chlorine and chlorine dioxide. Currently, there are several types of fuel cells.
Characteristics of the power supply system for UAVs:
Portable chargers based on hydrogen fuel cells:
Portable chargers based on hydrogen fuel cells are compact devices, comparable in weight and dimensions to existing battery chargers that are actively used in the world.
The ubiquitous portable technology in the modern world regularly needs to be recharged. Traditional portable systems are practically useless at low temperatures, and after performing their function they also require recharging using (electrical networks), which also reduces their efficiency and the autonomy of the device.
Each dihydrogen molecule acquires 2 electrons. The H ion moves from the anode to the cathode and causes an electric current by transferring an electron. What might fuel cells for airplanes look like? Today, tests are being carried out on aircraft to try to fly them using a lithium-ion hybrid fuel cell battery. The fuel cell's true benefit lies in its low-weight integrity: it is lighter, which helps reduce aircraft weight and therefore fuel consumption.
But for now, flying a fuel cell aircraft is not possible because it still has many drawbacks. Image of a fuel cell. What are the disadvantages of a fuel cell? First of all, if hydrogen were common, using it in large quantities would be problematic. Indeed, it is available not only on Earth. It is found in oxygen-containing water and ammonia. Therefore, it is necessary to electrolyze water to obtain it, and this is not yet a widespread method.
Hydrogen fuel cell systems require only the replacement of a compact fuel cartridge, after which the device is immediately ready for use.
Features of portable chargers:
Uninterruptible power supplies based on hydrogen fuel cells:
Guaranteed power supply systems based on hydrogen fuel cells are designed to organize backup power supply and temporary power supply. Guaranteed power supply systems based on hydrogen fuel cells offer significant advantages over traditional solutions for organizing temporary and backup power supply, using batteries and diesel generators.
Hydrogen is a gas, making it difficult to contain and transport. Another risk associated with the use of hydrogen is the risk of explosion, as it is a flammable gas. what supplies the battery for its production on a large scale requires another source of energy, be it oil, gas or coal, or nuclear energy, which makes its environmental balance significantly worse than kerosene and make heap, platinum, a metal that is even rarer and more expensive than gold.
The fuel cell provides energy by oxidizing the fuel at the anode and reducing the oxidizer at the cathode. The discovery of the fuel cell principle and the first implementations in the laboratory using sulfuric acid as an electrolyte is credited to chemist William Grove.

Characteristics of the uninterruptible power supply system:
Fuel cell is an electrochemical device similar to a galvanic cell, but differs from it in that the substances for the electrochemical reaction are supplied to it from the outside - in contrast to the limited amount of energy stored in a galvanic cell or battery.
Indeed, fuel cells have some advantages: those that use dihydrogen and dioxide only emit water vapor: it is therefore a clean technology. There are several types of fuel cells, depending on the nature of the electrolyte, the nature of the fuel, direct or indirect oxidation, and operating temperature.
The following table summarizes the main characteristics of these various devices. Several European programs are looking at other polymers, such as polybenzimidazole derivatives, which are more stable and cheaper. Battery compactness is also an ongoing challenge with membranes on the order of 15-50 microns, porous carbon anodes and stainless steel bipolar plates. Life expectancy can also be improved since, on the one hand, traces of carbon monoxide on the order of a few ppm in hydrogen are real poisons for the catalyst, and on the other hand, control of water in the polymer is mandatory.

Rice. 1. Some fuel cells
Fuel cells convert the chemical energy of fuel into electricity, bypassing ineffective combustion processes that occur with large losses. They convert hydrogen and oxygen into electricity through a chemical reaction. As a result of this process, water is formed and a large amount of heat is released. A fuel cell is very similar to a battery that can be charged and then use the stored electrical energy. The inventor of the fuel cell is considered to be William R. Grove, who invented it back in 1839. This fuel cell used a sulfuric acid solution as an electrolyte and hydrogen as a fuel, which was combined with oxygen in an oxidizing agent. Until recently, fuel cells were used only in laboratories and on spacecraft.

Unlike other power generators, such as internal combustion engines or turbines powered by gas, coal, fuel oil, etc., fuel cells do not burn fuel. This means no noisy high-pressure rotors, no loud exhaust noise, no vibrations. Fuel cells produce electricity through a silent electrochemical reaction. Another feature of fuel cells is that they convert the chemical energy of the fuel directly into electricity, heat and water.
Fuel cells are highly efficient and do not produce large amounts of greenhouse gases such as carbon dioxide, methane and nitrous oxide. The only emissions from fuel cells are water in the form of steam and a small amount of carbon dioxide, which is not released at all if pure hydrogen is used as fuel. Fuel cells are assembled into assemblies and then into individual functional modules.
Fuel cells have no moving parts (at least not within the cell itself) and therefore do not obey Carnot's law. That is, they will have greater than 50% efficiency and are especially effective at low loads. Thus, fuel cell vehicles can become (and have already proven to be) more fuel efficient than conventional vehicles in real-world driving conditions.
The fuel cell produces a constant voltage electric current that can be used to drive the electric motor, lighting, and other electrical systems in the vehicle.
There are several types of fuel cells, differing in the chemical processes used. Fuel cells are usually classified by the type of electrolyte they use.
Some types of fuel cells are promising for power plant propulsion, while others are promising for portable devices or to drive cars.
1. Alkaline fuel cells (ALFC)
Alkaline fuel cell- This is one of the very first elements developed. Alkaline fuel cells (AFC) are one of the most studied technologies, used since the mid-60s of the twentieth century by NASA in the Apollo and Space Shuttle programs. On board these spacecraft, fuel cells produce electrical energy and potable water.

Alkaline fuel cells are one of the most efficient cells used to generate electricity, with power generation efficiency reaching up to 70%.
Alkaline fuel cells use an electrolyte, i.e. water solution potassium hydroxide contained in a porous stabilized matrix. The potassium hydroxide concentration may vary depending on the operating temperature of the fuel cell, which ranges from 65°C to 220°C. The charge carrier in SHTE is the hydroxyl ion (OH-), moving from the cathode to the anode, where it reacts with hydrogen, producing water and electrons. The water produced at the anode moves back to the cathode, again generating hydroxyl ions there. As a result of this series of reactions taking place in the fuel cell, electricity and, as a by-product, heat are produced:
Reaction at the anode: 2H2 + 4OH- => 4H2O + 4e
Reaction at the cathode: O2 + 2H2O + 4e- => 4OH
General reaction systems: 2H2 + O2 => 2H2O
The advantage of SHTE is that these fuel cells are the cheapest to produce, since the catalyst needed on the electrodes can be any of the substances that are cheaper than those used as catalysts for other fuel cells. In addition, SHTEs operate at relatively low temperatures and are among the most efficient.
One of the characteristic features of SHTE is its high sensitivity to CO2, which may be contained in fuel or air. CO2 reacts with the electrolyte, quickly poisons it, and greatly reduces the efficiency of the fuel cell. Therefore, the use of SHTE is limited to enclosed spaces, such as space and underwater vehicles; they operate on pure hydrogen and oxygen.
2. Molten carbonate fuel cells (MCFC)
Fuel cells with molten carbonate electrolyte are high temperature fuel cells. The high operating temperature allows the direct use of natural gas without a fuel processor and low calorific value fuel gas from industrial processes and other sources. This process was developed in the mid-60s of the twentieth century. Since then, production technology, performance and reliability have been improved.

The operation of RCFC differs from other fuel cells. These cells use an electrolyte made from a mixture of molten carbonate salts. Currently, two types of mixtures are used: lithium carbonate and potassium carbonate or lithium carbonate and sodium carbonate. To melt carbonate salts and achieve a high degree of ion mobility in the electrolyte, fuel cells with molten carbonate electrolyte operate at high temperatures (650°C). Efficiency varies between 60-80%.
When heated to a temperature of 650°C, the salts become a conductor for carbonate ions (CO32-). These ions pass from the cathode to the anode, where they combine with hydrogen to form water, carbon dioxide and free electrons. These electrons are sent through an external electrical circuit back to the cathode, generating electric current and heat as a by-product.
Reaction at the anode: CO32- + H2 => H2O + CO2 + 2e
Reaction at the cathode: CO2 + 1/2O2 + 2e- => CO32-
General reaction of the element: H2(g) + 1/2O2(g) + CO2(cathode) => H2O(g) + CO2(anode)
The high operating temperatures of molten carbonate electrolyte fuel cells have certain advantages. The advantage is the ability to use standard materials (stainless steel sheets and nickel catalyst on the electrodes). The waste heat can be used to produce high pressure steam. High reaction temperatures in the electrolyte also have their advantages. The use of high temperatures requires a long time to achieve optimal operating conditions, and the system responds more slowly to changes in energy consumption. These characteristics allow the use of fuel cell installations with molten carbonate electrolyte under constant power conditions. High temperatures prevent damage to the fuel cell by carbon monoxide, “poisoning,” etc.
Fuel cells with molten carbonate electrolyte are suitable for use in large stationary installations. Thermal power plants with an electrical output power of 2.8 MW are commercially produced. Installations with output power up to 100 MW are being developed.
3. Phosphoric acid fuel cells (PAFC)
Fuel cells based on phosphoric (orthophosphoric) acid became the first fuel cells for commercial use. This process was developed in the mid-60s of the twentieth century, tests have been carried out since the 70s of the twentieth century. The result was increased stability and performance and reduced cost.

Phosphoric (orthophosphoric) acid fuel cells use an orthophosphoric acid (H3PO4) electrolyte at concentrations up to 100%. The ionic conductivity of phosphoric acid is low at low temperatures, so these fuel cells are used at temperatures up to 150-220 °C.
The charge carrier in fuel cells of this type is hydrogen (H+, proton). A similar process occurs in proton exchange membrane fuel cells (PEMFCs), in which hydrogen supplied to the anode is split into protons and electrons. Protons travel through the electrolyte and combine with oxygen from the air at the cathode to form water. The electrons are sent through an external electrical circuit, thereby generating an electric current. Below are reactions that generate electric current and heat.
Reaction at the anode: 2H2 => 4H+ + 4e
Reaction at the cathode: O2(g) + 4H+ + 4e- => 2H2O
General reaction of the element: 2H2 + O2 => 2H2O
The efficiency of fuel cells based on phosphoric (orthophosphoric) acid is more than 40% when generating electrical energy. With combined production of heat and electricity, the overall efficiency is about 85%. In addition, given operating temperatures, waste heat can be used to heat water and generate atmospheric pressure steam.
The high performance of thermal power plants using fuel cells based on phosphoric (orthophosphoric) acid in the combined production of thermal and electrical energy is one of the advantages of this type of fuel cells. The units use carbon monoxide with a concentration of about 1.5%, which significantly expands the choice of fuel. Simple design, low degree of electrolyte volatility and increased stability are also advantages of such fuel cells.
Thermal power plants with electrical output power of up to 400 kW are commercially produced. Installations with a capacity of 11 MW have passed appropriate tests. Installations with output power up to 100 MW are being developed.
4. Proton exchange membrane fuel cells (PEMFCs)
Proton exchange membrane fuel cells are considered the most the best type fuel cells to generate power for vehicles, which can replace gasoline and diesel internal combustion engines. These fuel cells were first used by NASA for the Gemini program. Installations based on MOPFC with power from 1 W to 2 kW have been developed and demonstrated.

The electrolyte in these fuel cells is a solid polymer membrane (a thin film of plastic). When saturated with water, this polymer allows protons to pass through but does not conduct electrons.
The fuel is hydrogen, and the charge carrier is a hydrogen ion (proton). At the anode, the hydrogen molecule is split into a hydrogen ion (proton) and electrons. Hydrogen ions pass through the electrolyte to the cathode, and electrons move around the outer circle and produce electrical energy. Oxygen, which is taken from the air, is supplied to the cathode and combines with electrons and hydrogen ions to form water. The following reactions occur at the electrodes: Reaction at the anode: 2H2 + 4OH- => 4H2O + 4eReaction at the cathode: O2 + 2H2O + 4e- => 4OH Overall cell reaction: 2H2 + O2 => 2H2O Compared to other types of fuel cells, fuel cells with a proton exchange membrane produce more energy for a given volume or weight of the fuel cell. This feature allows them to be compact and lightweight. In addition, the operating temperature is less than 100°C, which allows you to quickly start operation. These characteristics, as well as the ability to quickly change energy output, are just a few that make these fuel cells a prime candidate for use in vehicles.
Another advantage is that the electrolyte is a solid and not liquid substance. It is easier to retain gases at the cathode and anode using a solid electrolyte, so such fuel cells are cheaper to produce. With a solid electrolyte, there are no orientation issues and fewer corrosion problems, increasing the longevity of the cell and its components.

5. Solid oxide fuel cells (SOFC)
Solid oxide fuel cells are the highest operating temperature fuel cells. The operating temperature can vary from 600°C to 1000°C, allowing the use of different types of fuel without special pre-treatment. To handle such high temperatures, the electrolyte used is a thin solid metal oxide on a ceramic base, often an alloy of yttrium and zirconium, which is a conductor of oxygen ions (O2-). The technology of using solid oxide fuel cells has been developing since the late 50s of the twentieth century and has two configurations: planar and tubular.
The solid electrolyte provides a sealed transition of gas from one electrode to another, while liquid electrolytes are located in a porous substrate. The charge carrier in fuel cells of this type is the oxygen ion (O2-). At the cathode, oxygen molecules from the air are separated into an oxygen ion and four electrons. Oxygen ions pass through the electrolyte and combine with hydrogen, creating four free electrons. The electrons are sent through an external electrical circuit, generating electric current and waste heat.

Reaction at the anode: 2H2 + 2O2- => 2H2O + 4e
Reaction at the cathode: O2 + 4e- => 2O2-
General reaction of the element: 2H2 + O2 => 2H2O
The efficiency of electrical energy production is the highest of all fuel cells - about 60%. In addition, high operating temperatures allow for the combined production of thermal and electrical energy to generate high-pressure steam. Combining a high-temperature fuel cell with a turbine makes it possible to create a hybrid fuel cell to increase the efficiency of generating electrical energy by up to 70%.
Solid oxide fuel cells operate at very high temperatures (600°C-1000°C), resulting in significant time required to reach optimal operating conditions and a slower system response to changes in energy consumption. At such high operating temperatures, no converter is required to recover hydrogen from the fuel, allowing the thermal power plant to operate with relatively impure fuels resulting from gasification of coal or waste gases, etc. The fuel cell is also excellent for high power applications, including industrial and large central power plants. Modules with an electrical output power of 100 kW are commercially produced.
6. Direct methanol oxidation fuel cells (DOMFC)
Direct methanol oxidation fuel cells They are successfully used in the field of powering mobile phones, laptops, as well as to create portable power sources, which is what the future use of such elements is aimed at.
The design of fuel cells with direct oxidation of methanol is similar to the design of fuel cells with a proton exchange membrane (MEPFC), i.e. A polymer is used as an electrolyte, and a hydrogen ion (proton) is used as a charge carrier. But liquid methanol (CH3OH) oxidizes in the presence of water at the anode, releasing CO2, hydrogen ions and electrons, which are sent through an external electrical circuit, thereby generating an electric current. Hydrogen ions pass through the electrolyte and react with oxygen from the air and electrons from the external circuit to form water at the anode.
Reaction at the anode: CH3OH + H2O => CO2 + 6H+ + 6eReaction at the cathode: 3/2O2 + 6H+ + 6e- => 3H2O General reaction of the element: CH3OH + 3/2O2 => CO2 + 2H2O The development of such fuel cells has been carried out since the beginning of the 90s s of the twentieth century and their specific power and efficiency were increased to 40%.
These elements were tested in the temperature range of 50-120°C. Because of their low operating temperatures and the absence of the need for a converter, such fuel cells are a prime candidate for use in mobile phones and other consumer products, as well as in car engines. Their advantage is also their small size.
7. Polymer electrolyte fuel cells (PEFC)

In the case of polymer electrolyte fuel cells, the polymer membrane consists of polymer fibers with water regions in which conduction water ions H2O+ (proton, red) attaches to a water molecule. Water molecules pose a problem due to slow ion exchange. Therefore, a high concentration of water is required both in the fuel and at the outlet electrodes, which limits the operating temperature to 100°C.
8. Solid acid fuel cells (SFC)

In solid acid fuel cells, the electrolyte (CsHSO4) does not contain water. The operating temperature is therefore 100-300°C. The rotation of the SO42 oxyanions allows the protons (red) to move as shown in the figure. Typically, a solid acid fuel cell is a sandwich in which a very thin layer of solid acid compound is sandwiched between two electrodes that are tightly pressed together to ensure good contact. When heated, the organic component evaporates, exiting through the pores in the electrodes, maintaining the ability of multiple contacts between the fuel (or oxygen at the other end of the element), the electrolyte and the electrodes.

9. Comparison of the most important characteristics of fuel cells
Fuel cell type | Operating temperature | Power generation efficiency | Fuel type | Scope of application |
Medium and large installations |
||||
Pure hydrogen | installations |
|||
Pure hydrogen | Small installations |
|||
Most hydrocarbon fuels | Small, medium and large installations |
|||
Portable installations |
||||
Pure hydrogen | Space researched |
|||
Pure hydrogen | Small installations |

10. Use of fuel cells in cars



Prepare everything you need. To make a simple fuel cell, you will need 12 inches of platinum or platinum-coated wire, a popsicle stick, a 9-volt battery and battery holder, clear tape, a glass of water, table salt (optional), a thin metal rod, and a voltmeter.
- A 9-volt battery and battery holder can be purchased at an electronics or hardware store.
Cut two pieces of 15 centimeters long from platinum or platinum-coated wire. Platinum wire is used for special purposes and can be purchased at an electronics store. It will serve as a catalyst for the reaction.
Wrap pieces of wire around a thin metal rod to create the shape of springs. These will be the electrodes of the fuel cell. Grab the end of the wire and wind it tightly around the rod to create a coil spring. Remove the first wire from the rod and wind the second piece of wire.
- You can use a nail, a wire hanger, or a tester probe as a rod for winding the wire.
Cut the battery holder wires in half. Take the wire cutters, cut both wires attached to the holder in half and remove the insulation from them. You will attach these bare wires to the electrodes.
- Using the appropriate part of the wire cutters, strip the insulation from the ends of the wire. Strip the insulation from the ends of the wires you cut from the battery holder.
- Cut wire under adult supervision.
Attach the ends of the wires, stripped of insulation, to the electrodes. Connect the wires to the electrodes so that you can then connect a power source (battery holder) and a voltmeter to determine how much voltage the fuel cell is producing.
- Twist the red battery holder wire and the cut red wire around the top end of one of the wire spools, leaving most of it free.
- Wrap the top end of the second coil with the black battery holder wire and the cut black wire.
Attach the electrodes to a popsicle stick or wooden rod. The popsicle stick should be longer than the neck of the glass of water so that it can rest on top of it. Glue the electrodes so that they hang down from the stick and fall into the water.
- You can use clear tape or electrical tape. The main thing is that the electrodes are securely attached to the stick.
Pour tap or salt water into a glass. For the reaction to occur, water must contain electrolytes. Distilled water is not suitable for this, since it does not contain impurities that can serve as electrolytes. For the chemical reaction to proceed normally, you can dissolve salt or baking soda in water.
- Regular tap water also contains mineral impurities, so it can be used as an electrolyte if you don't have salt on hand.
- Add salt or baking soda at the rate of one tablespoon (20 grams) per glass of water. Stir the water until the salt or baking soda is completely dissolved.
Place a stick with electrodes on the neck of a glass of water. In this case, the electrodes in the form of wire springs should be submerged under water for most of their length, with the exception of contacts with the wires of the battery holder. Only the platinum wire should be under water.
- If necessary, secure the stick with tape to keep the electrodes in the water.
Connect the wires coming from the electrodes to a voltmeter or LED light bulb. Using a voltmeter, you can determine the voltage produced by the activated fuel cell. Connect the red wire to the positive terminal and the black wire to the negative terminal of the voltmeter.
- At this stage, the voltmeter may show a small value, for example 0.01 volts, although the voltage across it should be zero.
- You can also connect a small light bulb, such as a flashlight or LED.