Carboxylic acids are organic acids. They are part of living organisms and participate in metabolism. Chemical properties carboxylic acids are caused by the presence of the carboxyl group -COOH. These include acetic, formic, oxalic, butyric and a number of other acids.
General description
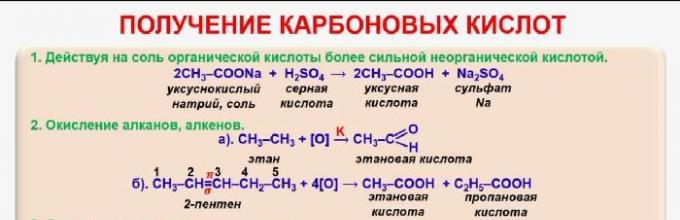
There are several ways to obtain carboxylic acids:
- oxidation of alcohols - C 2 H 5 OH + O2 → CH 3 COOH + H 2 O (acetic acid is formed from ethanol);
- oxidation of aldehydes - CH 3 COH + [O] → CH 3 COOH;
- butane oxidation - 2C 4 H 10 + 5O 2 → 4CH 3 COOH + 2H 2 O;
- alcohol carbonylation - CH 3 + CO → CH 3 COOH;
- decomposition of oxalic acid to produce formic acid - C 2 H 2 O 4 → HCOOH + CO 2;
- interaction of salts with concentrated sulfuric acid - CH 3 COONa + H 2 SO 4 → CH 3 COOH + NaHSO 4.

Rice. 1. Methods for producing carboxylic acids.
Physical properties of carboxylic acids:
- boiling point higher than that of corresponding hydrocarbons and alcohols;
- good solubility in water - dissolve into hydrogen cations and anions of the acid residue (they are weak electrolytes);
- increasing the number of carbon atoms decreases the strength of acids.
Carboxylic acids have strong hydrogen bonds (stronger than alcohols), which is caused by the high positive charge on the hydrogen atom in the carboxyl group.
Interaction
Carboxylic acids change the color of indicators. Litmus and methyl orange turn red.

Rice. 2. Interaction with indicators.
In the table chemical properties carboxylic acids describes the interaction of acids with other substances.
|
Reactions |
Result |
Example |
|
With metals |
Hydrogen is released and salts are formed |
2CH 3 COOH + Mg → (CH 3 COO) 2 Mg + H 2 |
|
With oxides |
Salt and water are formed |
2CH 3 COOH + ZnO → (CH 3 COO) 2 Zn + H 2 O |
|
With grounds (neutralization) |
Salt and water are formed |
CH 3 COOH + NaOH → CH 3 COONa + H 2 O |
|
With carbonates |
Carbon dioxide and water are released |
2CH 3 COOH + CaCO 3 → (CH 3 COO) 2 Ca + H 2 O + CO 2 |
|
With salts of weak acids |
Inorganic acid is formed |
2CH 3 COOH + Na 2 SiO 3 → 2CH 3 COONa + H 2 SiO 3 |
|
With ammonia or ammonium hydroxide |
Ammonium acetate is formed. When interacting with hydroxide, water is released |
CH 3 COOH + NH 3 → CH 3 COONH 4 CH 3 COOH + NH 4 OH → CH 3 COONH 4 + H 2 O |
|
With alcohols (esterification) |
Esters are formed |
CH 3 COOH + C 2 H 5 OH → CH 3 COOC 2 H 5 + H 2 O |
|
Halogenation |
Salt is formed |
CH 3 COOH + Br 2 → CH 2 BrCOOH |
Salts formed by the interaction of substances with formic acid are called formates, and with acetic acid - acetates.
Decarboxylation
The removal of a carboxyl group is called decarboxylation, which occurs in the following cases:
- when heating salts in the presence of solid alkalis with the formation of alkanes - RCOONa solid + NaOH solid → RH + Na 2 CO 3;
- when heated solid salts- (CH 3 COO) 2 Ca → CH 3 -CO-CH 3 + CaCO 3;
- when calcining benzoic acid - Ph-COOH → PhH + CO 2;
- during electrolysis of salt solutions - 2RCOONa + H 2 O → R-R + 2CO 2 + 2NaOH.
Carboxylic acids Compounds that contain a carboxyl group are called:
Carboxylic acids are distinguished:
- monobasic carboxylic acids;
- dibasic (dicarboxylic) acids (2 groups UNS).
Depending on their structure, carboxylic acids are distinguished:
- aliphatic;
- alicyclic;
- aromatic.
Examples of carboxylic acids.


Preparation of carboxylic acids.
1. Oxidation of primary alcohols with potassium permanganate and potassium dichromate:

2. Hybrolysis of halogen-substituted hydrocarbons containing 3 halogen atoms per carbon atom:
3. Preparation of carboxylic acids from cyanides:
When heated, the nitrile hydrolyzes to form ammonium acetate:
When acidified, acid precipitates:
4. Use of Grignard reagents:
5. Hydrolysis esters:
6. Hydrolysis of acid anhydrides:
7. Specific methods for producing carboxylic acids:
Formic acid is produced by heating carbon(II) monoxide with powdered sodium hydroxide under pressure:
Acetic acid is produced by the catalytic oxidation of butane with atmospheric oxygen:
Benzoic acid is obtained by oxidation of monosubstituted homologues with a solution of potassium permanganate:
Canniciaro's reaction. Benzaldehyde is treated with 40-60% sodium hydroxide solution at room temperature.

Chemical properties of carboxylic acids.
IN aqueous solution carboxylic acids dissociate:

The equilibrium is shifted strongly to the left, because carboxylic acids are weak.
Substituents affect acidity due to an inductive effect. Such substituents pull electron density towards themselves and a negative inductive effect (-I) occurs on them. The withdrawal of electron density leads to an increase in the acidity of the acid. Electron-donating substituents create a positive inductive charge.

1. Formation of salts. Reaction with basic oxides, salts of weak acids and active metals:

Carboxylic acids are weak, because mineral acids displace them from the corresponding salts:
2. Formation of functional derivatives of carboxylic acids:
3. Esters when heating an acid with an alcohol in the presence of sulfuric acid - esterification reaction:
4. Formation of amides, nitriles:
3. The properties of acids are determined by the presence of a hydrocarbon radical. If the reaction occurs in the presence of red phosphorus, the following product is formed:
4. Addition reaction.

8. Decarboxylation. The reaction is carried out by fusing alkali with salt alkali metal carboxylic acid:
9. Dibasic acid is easily eliminated CO 2 when heated:
Additional materials on the topic: Carboxylic acids.
Chemistry calculators |
|
| Chemistry online on our website to solve problems and equations. | |
The oxidation of saturated hydrocarbons with oxygen on special catalysts to carboxylic acids is carried out in industry, but this method does not differ in selectivity. Typically, mixtures of carboxylic acids are obtained because oxidation breaks various carbon-carbon bonds.
The oxidation of alkenes with strong oxidizing agents is much more selective. When alkenes, which have one hydrogen atom at each carbon atom of a double bond, are heated with an alkaline solution of potassium permanganate, a mixture of two carboxylic acids is formed. If the alkene is symmetrical, then two molecules of one carboxylic acid are formed. The same oxidation can be carried out by heating alkenes with concentrated nitric acid.
![]()
Similarly, when alkynes are oxidized with an alkaline solution of potassium permanganate, carboxylic acids are obtained. For example, acetic acid can be obtained by oxidizing either 2-butene or 2-butine.

Alkylbenzenes are oxidized to benzoic acid either with oxygen on catalysts (in industry) or by heating with potassium permanganate. For example, boiling toluene with an aqueous solution of potassium permanganate and then acidifying the solution leads to benzoic acid.

Carboxylic acids can also be obtained by oxidation of primary alcohols or aldehydes. Chromium compounds in the highest oxidation state are usually used as oxidizing agents, for example, chromic anhydride, potassium permanganate in an alkaline medium, concentrated nitric acid. Aldehydes are easily oxidized by other oxidizing agents, for example, an ammonia solution of silver oxide (the “silver mirror” reaction).

1.2. Synthesis of carboxylic acids from halogen derivatives
1.2.1.Synthesis of carboxylic acids via nitriles
Alkyl halides react with sodium cyanide to form alkyl cyanides, which are nitriles of carboxylic acids. The latter are hydrolyzed in an acidic environment to carboxylic acids.

Thus, a two-stage replacement of the halogen atom in the halogen derivative molecule with a carboxyl group occurs. Thus, to obtain valeric acid (5 carbon atoms), it is necessary to start from butyl halide.
1-bromobutane nitrile valeric acid
valeric acid
1.2.2. Synthesis of carboxylic acids by the Grignard reaction
Grignard reagents, which are prepared from halogen derivatives by reaction with magnesium metal, are nucleophilic reagents. Therefore, to synthesize carboxylic acids from them, a carboxylation reaction is used with the help of electrophilic carbon dioxide.
To obtain benzoic acid by this method, it is necessary to take, for example, bromobenzene as the starting halogen derivative, which by reaction with magnesium, subsequent reaction of phenylmagnesium bromide with carbon dioxide and final hydrolysis of the magnesium salt is converted into benzoic acid.
1.3. Hydrolysis of carboxylic acid derivatives
Like nitriles and salts, the hydrolysis of which has already been discussed, other derivatives of carboxylic acids are hydrolyzed to carboxylic acids. Reactions can be catalyzed by both acids and alkalis. For example, the hydrolysis of propanoic acid methyl ester catalyzed by a strong mineral acid produces propanoic acid and methanol.
When acetanilide (acetic acid phenylamide) is heated with an aqueous solution of sodium hydroxide, sodium acetate and aniline are obtained.
Hydrolysis of the benzoic anhydride molecule results in the formation of two benzoic acid molecules.

Catalysis and the mechanism of hydrolysis will be discussed in more detail in the sections devoted to carboxylic acid derivatives
Preparation of carboxylic acids
I. In industry
1. Isolated from natural products
(fats, waxes, essential and vegetable oils)
2. Oxidation of alkanes:
2CH 4 + + 3O 2 t,kat→ 2HCOOH + 2H 2 O
methane formic acid
2CH 3 -CH 2 -CH 2 -CH 3 + 5O 2 t,kat,p→4CH 3 COOH + 2H 2 O
n-butaneacetic acid
3. Oxidation of alkenes:
CH 2 = CH 2 + O 2 t,kat→CH3COOH
ethylene
WITH H 3 -CH=CH 2 + 4[O] t,kat→ CH 3 COOH + HCOOH (acetic acid + formic acid )
4. Oxidation of benzene homologues (production of benzoic acid):
C 6 H 5 -C n H 2n+1 + 3n[O] KMnO4,H+→ C 6 H 5 -COOH + (n-1)CO 2 + nH 2 O
5C 6 H 5 -CH 3 + 6KMnO 4 + 9H 2 SO 4 → 5C 6 H 5 -COOH + 3K 2 SO 4 + 6MnSO 4 + 14H 2 O
toluenebenzoic acid
5.Obtaining formic acid:
Stage 1: CO+NaOH t , p→HCOONa (sodium formate – salt )
2 stage: HCOONa + H 2 SO 4 → HCOOH + NaHSO 4
6. Preparation of acetic acid:
CH3OH+CO t,p→CH3COOH
Methanol
II. In the laboratory
1. Hydrolysis of esters:
2. From salts of carboxylic acids :
R-COONa + HCl → R-COOH + NaCl
3. Dissolving carboxylic acid anhydrides in water:
(R-CO) 2 O + H 2 O → 2 R-COOH
4. Alkaline hydrolysis of halogen derivatives of carboxylic acids:

III. General methods for preparing carboxylic acids
1. Oxidation of aldehydes:
R-COH + [O] → R-COOH
For example, the “Silver Mirror” reaction or oxidation with copper (II) hydroxide - qualitative reactions aldehydes
2. Oxidation of alcohols:
R-CH 2 -OH + 2[O] t,kat→ R-COOH + H 2 O
3. Hydrolysis of halogenated hydrocarbons containing three halogen atoms per carbon atom.

4. From cyanides (nitriles) - the method allows you to increase the carbon chain:
WITH H 3 -Br + Na-C≡N → CH 3 -CN + NaBr
CH3-CN - methyl cyanide (acetic acid nitrile)
WITH H 3 -CN + 2H 2 O t→ CH 3 COONH 4
acetate ammonium
CH 3 COONH 4 + HCl → CH 3 COOH + NH 4 Cl
5. Usage reagent Grignard
R-MgBr + CO 2 →R-COO-MgBr H2O→ R-COOH + Mg(OH)Br
APPLICATION OF CARBOXYLIC ACIDS
Formic acid– in medicine - formic alcohol (1.25% alcohol solution of formic acid), in beekeeping, in organic synthesis, in the production of solvents and preservatives; as a strong reducing agent.
Acetic acid– in the food and chemical industries (production of cellulose acetate, from which acetate fiber, organic glass, film are produced; for the synthesis of dyes, medicines and esters). In the household as a flavoring and preservative substance.
Butyric acid– for the production of flavoring additives, plasticizers and flotation reagents.
Oxalic acid– in the metallurgical industry (descaling).
Stearic C17H35COOH and palmitic acid C 15 H 31 COOH – as surfactants, lubricants in metalworking.
Oleic acid C 17 H 33 COOH is a flotation reagent and collector for the enrichment of non-ferrous metal ores.
Individual representatives
monobasic saturated carboxylic acids
Formic acid
was first isolated in the 17th century from red forest ants. Also found in stinging nettle juice. Anhydrous formic acid is a colorless liquid with a pungent odor and pungent taste that causes burns on the skin. It is used in the textile industry as a mordant for dyeing fabrics, for tanning leather, and also for various syntheses.
Acetic acid
widespread in nature - found in animal excretions (urine, bile, feces) and plants (green leaves). It is formed during fermentation, rotting, souring of wine, beer, and is found in sour milk and cheese. The melting point of anhydrous acetic acid is + 16.5°C, its crystals are as transparent as ice, which is why it is called glacial acetic acid. It was first obtained at the end of the 18th century by the Russian scientist T. E. Lovitz. Natural vinegar contains about 5% acetic acid. Vinegar essence is prepared from it, used in food industry for canning vegetables, mushrooms, fish. Acetic acid is widely used in the chemical industry for various syntheses.
Representatives of aromatic and unsaturated carboxylic acids
Benzoic acid
C 6 H 5 COOH is the most important representative of aromatic acids. Distributed in nature in flora: in balms, incense, essential oils. In animal organisms it is found in the breakdown products of protein substances. This crystalline substance, melting point 122°C, easily sublimes. It dissolves poorly in cold water. It dissolves well in alcohol and ether.
Unsaturated unsaturated acids with one double bond in the molecule have the general formula C n H 2 n -1 COOH.
High molecular weight unsaturated acids
often mentioned by nutritionists (they call them unsaturated). The most common of them is oleic
CH 3 –(CH 2) 7 –CH=CH–(CH 2) 7 –COOH or C 17 H 33 COOH. It is a colorless liquid that hardens in the cold.
Polyunsaturated acids with several double bonds are especially important: linoleic
CH 3 –(CH 2) 4 –(CH=CH–CH 2) 2 –(CH 2) 6 –COOH or C 17 H 31 COOH with two double bonds, linolenic
CH 3 –CH 2 –(CH=CH–CH 2) 3 –(CH 2) 6 –COOH or C 17 H 29 COOH with three double bonds and arachidonic
CH 3 –(CH 2) 4 –(CH=CH–CH 2) 4 –(CH 2) 2 –COOH with four double bonds; they are often called essential fatty acids. It is these acids that have the greatest biological activity: they participate in the transfer and metabolism of cholesterol, the synthesis of prostaglandins and other vital substances, and maintain the structure cell membranes, are necessary for the functioning of the visual apparatus and nervous system, affect the immune system. The absence of these acids in food inhibits the growth of animals, inhibits their reproductive function, and causes various diseases. The human body cannot synthesize linoleic and linolenic acids itself and must receive them ready-made with food (like vitamins). For synthesis arachidonic acid The body needs linoleic acid. Polyunsaturated fatty acids with 18 carbon atoms in the form of glycerol esters are found in the so-called drying oils - flaxseed, hemp, poppy, etc. Linoleic acid C17H31COOH and linolenic acid C 17 H 29 COOH are part of vegetable oils. For example, flaxseed oil contains about 25% linoleic acid and up to 58% linolenic acid.
Sorbic acid (2,4-hexadienoic) acid CH 3 –CH=CH–CH=CHCOOH was obtained from rowan berries (in Latin – sorbus). This acid is an excellent preservative, so rowan berries do not become moldy.
The simplest unsaturated acid, acrylic CH 2 = CHCOOH, has a pungent odor (in Latin acris - pungent, pungent). Acrylates (esters of acrylic acid) are used to produce organic glass, and its nitrile (acrylonitrile) is used to produce synthetic fibers.
When naming newly isolated acids, chemists often give free rein to their imagination. Thus, the name of the closest homologue of acrylic acid, croton
CH 3 – CH = CH – COOH, does not come from a mole at all, but from a plant Croton tiglium, from whose oil it was isolated. The synthetic isomer of crotonic acid is very important - methacrylic acid CH 2 = C (CH 3) – COOH, from the ester of which (methyl methacrylate), as well as from methyl acrylate, transparent plastic is made - plexiglass.
Unsaturated carbon acids are capable of addition reactions:
CH 2 = CH-COOH + H 2 → CH 3 -CH 2 -COOH
CH 2 =CH-COOH + Cl 2 → CH 2 Cl -CHCl -COOH
VIDEO:
CH 2 =CH-COOH + HCl → CH 2 Cl -CH 2 -COOH
CH 2 = CH-COOH + H 2 O → HO-CH 2 -CH 2 -COOH
The last two reactions proceed against Markovnikov's rule.
Unsaturated carboxylic acids and their derivatives are capable of polymerization reactions.