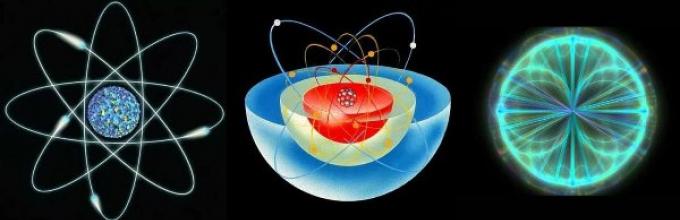
Composition of the atom.
An atom is made up of atomic nucleus And electron shell .
The nucleus of an atom consists of protons ( p+) and neutrons ( n 0). Most hydrogen atoms have a nucleus consisting of one proton.
Number of protons N(p+) is equal to the nuclear charge ( Z) and the ordinal number of the element in the natural series of elements (and in the periodic table of elements).
N(p +) = Z
Sum of neutrons N(n 0), denoted simply by the letter N, and number of protons Z called mass number and is designated by the letter A.
A = Z + N
The electron shell of an atom consists of electrons moving around the nucleus ( e -).
Number of electrons N(e-) in the electron shell of a neutral atom is equal to the number of protons Z at its core.
The mass of a proton is approximately equal to the mass of a neutron and 1840 times more mass electron, so the mass of the atom is practically equal to the mass of the nucleus.
The shape of the atom is spherical. The radius of the nucleus is approximately 100,000 times smaller than the radius of the atom.
Chemical element- type of atoms (collection of atoms) with the same nuclear charge (with the same number of protons in the nucleus).
Isotope- a collection of atoms of the same element with the same number of neutrons in the nucleus (or a type of atom with the same number of protons and the same number of neutrons in the nucleus).
Different isotopes differ from each other in the number of neutrons in the nuclei of their atoms.
Designation of an individual atom or isotope: (E - element symbol), for example: .
Structure of the electron shell of an atom
Atomic orbital- state of an electron in an atom. The symbol for the orbital is . Each orbital has a corresponding electron cloud.
Orbitals of real atoms in the ground (unexcited) state are of four types: s, p, d And f.
Electronic cloud- the part of space in which an electron can be found with a probability of 90 (or more) percent.
Note: sometimes the concepts of “atomic orbital” and “electron cloud” are not distinguished, calling both “atomic orbital”.
The electron shell of an atom is layered. Electronic layer formed by electron clouds of the same size. The orbitals of one layer form electronic ("energy") level, their energies are the same for the hydrogen atom, but different for other atoms.
Orbitals of the same type are grouped into electronic (energy) sublevels:
s-sublevel (consists of one s-orbitals), symbol - .
p-sublevel (consists of three p
d-sublevel (consists of five d-orbitals), symbol - .
f-sublevel (consists of seven f-orbitals), symbol - .
The energies of orbitals of the same sublevel are the same.
When designating sublevels, the number of the layer (electronic level) is added to the sublevel symbol, for example: 2 s, 3p, 5d means s-sublevel of the second level, p-sublevel of the third level, d-sublevel of the fifth level.
The total number of sublevels at one level is equal to the level number n. The total number of orbitals at one level is equal to n 2. Accordingly, the total number of clouds in one layer is also equal to n 2 .
Designations: - free orbital (without electrons), - orbital with unpaired electron, - an orbital with an electron pair (with two electrons).
The order in which electrons fill the orbitals of an atom is determined by three laws of nature (the formulations are given in simplified terms):
1. The principle of least energy - electrons fill the orbitals in order of increasing energy of the orbitals.
2. The Pauli principle - there cannot be more than two electrons in one orbital.
3. Hund's rule - within a sublevel, electrons first fill empty orbitals (one at a time), and only after that they form electron pairs.
The total number of electrons in the electronic level (or electron layer) is 2 n 2 .
The distribution of sublevels by energy is expressed as follows (in order of increasing energy):
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p ...
This sequence is clearly expressed by an energy diagram:
The distribution of an atom's electrons across levels, sublevels, and orbitals (electronic configuration of an atom) can be depicted as an electron formula, an energy diagram, or, more simply, as a diagram of electron layers ("electron diagram").
Examples electronic structure atoms:
Valence electrons- electrons of the atom that can take part in the formation chemical bonds. For any atom, these are all the outer electrons plus those pre-outer electrons whose energy is greater than that of the outer ones. For example: the Ca atom has 4 outer electrons s 2, they are also valence; the Fe atom has 4 outer electrons s 2 but he has 3 d 6, therefore the iron atom has 8 valence electrons. Valence electronic formula of the calcium atom is 4 s 2, and iron atoms - 4 s 2 3d 6 .
Periodic table of chemical elements by D. I. Mendeleev
(natural system of chemical elements)
Periodic law chemical elements (modern formulation): properties of chemical elements, as well as simple and complex substances, formed by them, are periodically dependent on the value of the charge from atomic nuclei.
Periodic table- graphic expression of the periodic law.
Natural series of chemical elements- a series of chemical elements arranged according to the increasing number of protons in the nuclei of their atoms, or, what is the same, according to the increasing charges of the nuclei of these atoms. The atomic number of an element in this series is equal to the number of protons in the nucleus of any atom of this element.
The table of chemical elements is constructed by “cutting” the natural series of chemical elements into periods(horizontal rows of the table) and groupings (vertical columns of the table) of elements with a similar electronic structure of atoms.
Depending on the way you combine elements into groups, the table may be long-period(elements with the same number and type of valence electrons are collected into groups) and short period(elements with the same number of valence electrons are collected into groups).
The short-period table groups are divided into subgroups ( main And side), coinciding with the groups of the long-period table.
All atoms of elements of the same period have the same number of electron layers, equal to the period number.
Number of elements in periods: 2, 8, 8, 18, 18, 32, 32. Most of the elements of the eighth period were obtained artificially; the last elements of this period have not yet been synthesized. All periods except the first begin with an element forming alkali metal(Li, Na, K, etc.) and end with a noble gas forming element (He, Ne, Ar, Kr, etc.).
In the short-period table there are eight groups, each of which is divided into two subgroups (main and secondary), in the long-period table there are sixteen groups, which are numbered in Roman numerals with the letters A or B, for example: IA, IIIB, VIA, VIIB. Group IA of the long-period table corresponds to the main subgroup of the first group of the short-period table; group VIIB - secondary subgroup of the seventh group: the rest - similarly.
The characteristics of chemical elements naturally change in groups and periods.
In periods (with increasing serial number)
- nuclear charge increases
- the number of outer electrons increases,
- the radius of atoms decreases,
- the strength of the bond between electrons and the nucleus increases (ionization energy),
- electronegativity increases,
- oxidizing properties are enhanced simple substances("non-metallicity"),
- the reducing properties of simple substances weaken ("metallicity"),
- weakens the basic character of hydroxides and corresponding oxides,
- the acidic character of hydroxides and corresponding oxides increases.
In groups (with increasing serial number)
- nuclear charge increases
- the radius of atoms increases (only in A-groups),
- the strength of the bond between electrons and the nucleus decreases (ionization energy; only in A-groups),
- electronegativity decreases (only in A-groups),
- the oxidizing properties of simple substances weaken ("non-metallicity"; only in A-groups),
- the reducing properties of simple substances are enhanced ("metallicity"; only in A-groups),
- the basic character of hydroxides and corresponding oxides increases (only in A-groups),
- weakens the acidic character of hydroxides and corresponding oxides (only in A-groups),
- the stability of hydrogen compounds decreases (their reducing activity increases; only in A-groups).
Tasks and tests on the topic "Topic 9. "Structure of the atom. Periodic law and periodic system of chemical elements by D. I. Mendeleev (PSHE) "."
- Periodic law - Periodic law and structure of atoms grades 8–9
You must know: the laws of filling orbitals with electrons (the principle of least energy, the Pauli principle, Hund's rule), the structure of the periodic table of elements.You must be able to: determine the composition of an atom by the position of the element in the periodic table, and, conversely, find an element in the periodic system, knowing its composition; depict the structure diagram, electronic configuration of an atom, ion, and, conversely, determine the position of a chemical element in the PSCE from the diagram and electronic configuration; characterize the element and the substances it forms according to its position in the PSCE; determine changes in the radius of atoms, properties of chemical elements and the substances they form within one period and one main subgroup of the periodic system.
Example 1. Determine the number of orbitals in the third electron level. What are these orbitals?
To determine the number of orbitals, we use the formula N orbitals = n 2 where n- level number. N orbitals = 3 2 = 9. One 3 s-, three 3 p- and five 3 d-orbitals.Example 2. Determine which element's atom has electronic formula 1 s 2 2s 2 2p 6 3s 2 3p 1 .
In order to determine what element it is, you need to find out its atomic number, which is equal to the total number of electrons of the atom. In this case: 2 + 2 + 6 + 2 + 1 = 13. This is aluminum.After making sure that everything you need has been learned, proceed to completing the tasks. We wish you success.
Recommended reading:- O. S. Gabrielyan and others. Chemistry 11th grade. M., Bustard, 2002;
- G. E. Rudzitis, F. G. Feldman. Chemistry 11th grade. M., Education, 2001.
As you know, everything material in the Universe consists of atoms. An atom is the smallest unit of matter that carries its properties. In turn, the structure of the atom is made up of a magical trinity of microparticles: protons, neutrons and electrons.
Moreover, each of the microparticles is universal. That is, you cannot find two different protons, neutrons or electrons in the world. They are all absolutely similar to each other. And the properties of the atom will depend only on the quantitative composition of these microparticles in general structure atom.
For example, the structure of a hydrogen atom consists of one proton and one electron. The next most complex atom, helium, consists of two protons, two neutrons and two electrons. Lithium atom - made of three protons, four neutrons and three electrons, etc.
Atomic structure (from left to right): hydrogen, helium, lithiumAtoms combine to form molecules, and molecules combine to form substances, minerals, and organisms. The DNA molecule, which is the basis of all living things, is a structure assembled from the same three magical bricks of the universe as the stone lying on the road. Although this structure is much more complex.
Even more amazing facts are revealed when we try to take a closer look at the proportions and structure of the atomic system. It is known that an atom consists of a nucleus and electrons moving around it along a trajectory describing a sphere. That is, it cannot even be called a movement in the usual sense of the word. Rather, the electron is located everywhere and immediately within this sphere, creating an electron cloud around the nucleus and forming an electromagnetic field.
 Schematic representations of the structure of an atom
Schematic representations of the structure of an atom The nucleus of an atom consists of protons and neutrons, and almost all the mass of the system is concentrated in it. But at the same time, the nucleus itself is so small that if its radius is increased to a scale of 1 cm, then the radius of the entire atomic structure will reach hundreds of meters. Thus, everything that we perceive as dense matter consists of more than 99% of the energetic bonds between physical particles and less than 1% of the physical forms themselves.
But what are these physical forms? What are they made of, and how material are they? To answer these questions, let's take a closer look at the structures of protons, neutrons, and electrons. So, we descend one more step into the depths of the microworld - to the level of subatomic particles.
What does an electron consist of?
The smallest particle of an atom is an electron. An electron has mass but no volume. In the scientific concept, an electron does not consist of anything, but is a structureless point.
An electron cannot be seen under a microscope. It is visible only in the form electronic cloud, which looks like a blurry sphere around the atomic nucleus. At the same time, it is impossible to say with accuracy where the electron is at a moment in time. Instruments are capable of capturing not the particle itself, but only its energy trace. The essence of the electron is not embedded in the concept of matter. It is rather like some empty form that exists only in movement and due to movement.

No structure in the electron has yet been discovered. It is the same point particle as an energy quantum. In fact, an electron is energy, however, it is a more stable form of it than the one represented by photons of light.
At the moment, the electron is considered indivisible. This is understandable, because it is impossible to divide something that has no volume. However, the theory already has developments according to which the electron contains a trinity of such quasiparticles as:
- Orbiton – contains information about the orbital position of the electron;
- Spinon – responsible for spin or torque;
- Holon – carries information about the charge of the electron.
However, as we see, quasiparticles have absolutely nothing in common with matter, and carry only information.
 Photos of atoms different substances V electron microscope
Photos of atoms different substances V electron microscope
Interestingly, an electron can absorb energy quanta, such as light or heat. In this case, the atom moves to a new energy level, and the boundaries of the electron cloud expand. It also happens that the energy absorbed by an electron is so great that it can jump out of the atomic system and continue its movement as an independent particle. At the same time, it behaves like a photon of light, that is, it seems to cease to be a particle and begins to exhibit the properties of a wave. This was proven in an experiment.
Jung's experiment
During the experiment, a stream of electrons was directed at a screen with two slits cut in it. Passing through these slits, the electrons collided with the surface of another projection screen, leaving their mark on it. As a result of this “bombardment” of electrons, an interference pattern appeared on the projection screen, similar to the one that would appear if waves, but not particles, passed through two slits.
This pattern occurs because a wave passing between two slits is divided into two waves. As a result of further movement, the waves overlap each other, and in some areas they are mutually cancelled. The result is many fringes on the projection screen, instead of just one, as would be the case if the electron behaved like a particle.

Structure of the nucleus of an atom: protons and neutrons
Protons and neutrons make up the nucleus of an atom. And despite the fact that the core occupies less than 1% of the total volume, it is in this structure that almost the entire mass of the system is concentrated. But physicists are divided on the structure of protons and neutrons, and at the moment There are two theories at once.
- Theory No. 1 - Standard
The Standard Model says that protons and neutrons are made up of three quarks connected by a cloud of gluons. Quarks are point particles, just like quanta and electrons. And gluons are virtual particles that ensure the interaction of quarks. However, neither quarks nor gluons were ever found in nature, so this model is subject to severe criticism.
- Theory #2 - Alternative
But according to the alternative unified field theory developed by Einstein, the proton, like the neutron, like any other particle physical world, is an electromagnetic field rotating at the speed of light.
 Electromagnetic fields man and planet
Electromagnetic fields man and planet What are the principles of atomic structure?
Everything in the world - thin and dense, liquid, solid and gaseous - is just the energy states of countless fields that permeate the space of the Universe. The higher the energy level in the field, the thinner and less perceptible it is. The lower the energy level, the more stable and tangible it is. The structure of the atom, as well as the structure of any other unit of the Universe, lies in the interaction of such fields - different in energy density. It turns out that matter is just an illusion of the mind.
Electrons
The concept of atom arose in the ancient world to designate particles of matter. Translated from Greek, atom means “indivisible.”

The Irish physicist Stoney, based on experiments, came to the conclusion that electricity is transferred by the smallest particles existing in the atoms of all chemical elements. In 1891, Stoney proposed to call these particles electrons, which means “amber” in Greek. A few years after the electron got its name, the English physicist Joseph Thomson and the French physicist Jean Perrin proved that electrons carry a negative charge. This is the smallest negative charge, which in chemistry is taken as one (-1). Thomson even managed to determine the speed of the electron (the speed of the electron in the orbit is inversely proportional to the orbital number n. The radii of the orbits increase in proportion to the square of the orbital number. In the first orbit of the hydrogen atom (n=1; Z=1) the speed is ≈ 2.2·106 m/ s, that is, about a hundred times less than the speed of light c = 3·108 m/s) and the mass of the electron (it is almost 2000 times less than the mass of the hydrogen atom).

State of electrons in an atom
The state of an electron in an atom is understood as a set of information about the energy of a particular electron and the space in which it is located. An electron in an atom does not have a trajectory of motion, i.e. we can only talk about the probability of finding it in the space around the nucleus.
 It can be located in any part of this space surrounding the nucleus, and the totality of its various positions is considered as an electron cloud with a certain negative charge density. Figuratively, this can be imagined this way: if it were possible to photograph the position of an electron in an atom after hundredths or millionths of a second, as in a photo finish, then the electron in such photographs would be represented as dots. If countless such photographs were superimposed, the picture would be of an electron cloud with the greatest density where there would be the most of these points.
It can be located in any part of this space surrounding the nucleus, and the totality of its various positions is considered as an electron cloud with a certain negative charge density. Figuratively, this can be imagined this way: if it were possible to photograph the position of an electron in an atom after hundredths or millionths of a second, as in a photo finish, then the electron in such photographs would be represented as dots. If countless such photographs were superimposed, the picture would be of an electron cloud with the greatest density where there would be the most of these points.
The space around the atomic nucleus in which an electron is most likely to be found is called an orbital. It contains approximately 90% electronic cloud, and this means that about 90% of the time the electron is in this part of space. They are distinguished by shape 4 currently known types of orbitals, which are designated by Latin letters s, p, d and f. Graphic representation Some forms of electron orbitals are shown in the figure.

The most important characteristic of the motion of an electron in a certain orbital is energy of its connection with the nucleus. Electrons with similar energy values form a single electron layer, or energy level. Energy levels are numbered starting from the nucleus - 1, 2, 3, 4, 5, 6 and 7.
The integer n, indicating the number of the energy level, is called the principal quantum number. It characterizes the energy of electrons occupying a given energy level. Electrons of the first energy level, closest to the nucleus, have the lowest energy. Compared to electrons of the first level, electrons of subsequent levels will be characterized by a large supply of energy. Consequently, the electrons of the outer level are least tightly bound to the atomic nucleus.
The largest number of electrons at an energy level is determined by the formula:
N = 2n 2 ,
where N is the maximum number of electrons; n is the level number, or the main quantum number. Consequently, the first energy level closest to the nucleus can contain no more than two electrons; on the second - no more than 8; on the third - no more than 18; on the fourth - no more than 32.
Starting from the second energy level (n = 2), each of the levels is divided into sublevels (sublayers), slightly different from each other in the binding energy with the nucleus. The number of sublevels is equal to the value of the main quantum number: the first energy level has one sublevel; the second - two; third - three; fourth - four sublevels. The sublevels, in turn, are formed by orbitals. Each valuen corresponds to the number of orbitals equal to n.
Sublevels are usually denoted by Latin letters, as well as the shape of the orbitals of which they consist: s, p, d, f.

Protons and Neutrons

An atom of any chemical element is comparable to a tiny solar system. Therefore, this model of the atom, proposed by E. Rutherford, is called planetary.
The atomic nucleus, in which the entire mass of the atom is concentrated, consists of particles of two types - protons and neutrons.
Protons have a charge equal to the charge of electrons, but opposite in sign (+1), and a mass equal to mass hydrogen atom (it is taken as a unit in chemistry). Neutrons carry no charge, they are neutral and have a mass equal to the mass of a proton.
Protons and neutrons together are called nucleons (from the Latin nucleus - nucleus). The sum of the number of protons and neutrons in an atom is called the mass number. For example, the mass number of an aluminum atom is:

13 + 14 = 27
number of protons 13, number of neutrons 14, mass number 27
Since the mass of the electron, which is negligibly small, can be neglected, it is obvious that the entire mass of the atom is concentrated in the nucleus. Electrons are designated e - .
Since the atom electrically neutral, then it is also obvious that the number of protons and electrons in an atom is the same. It is equal to the serial number of the chemical element assigned to it in Periodic table. The mass of an atom consists of the mass of protons and neutrons. Knowing the atomic number of the element (Z), i.e. the number of protons, and the mass number (A), equal to the sum of the numbers of protons and neutrons, you can find the number of neutrons (N) using the formula:

N = A - Z
For example, the number of neutrons in an iron atom is:
56 — 26 = 30
Isotopes
Varieties of atoms of the same element that have the same nuclear charge but different mass numbers are called isotopes. Chemical elements found in nature are a mixture of isotopes. Thus, carbon has three isotopes with masses 12, 13, 14; oxygen - three isotopes with masses 16, 17, 18, etc. The relative atomic mass of a chemical element usually given in the Periodic Table is the average value of the atomic masses of a natural mixture of isotopes of a given element, taking into account their relative abundance in nature. Chemical properties The isotopes of most chemical elements are exactly the same. However, hydrogen isotopes vary greatly in properties due to the dramatic multiple increase in their relative atomic mass; they are even given individual names and chemical symbols.

Elements of the first period
Diagram of the electronic structure of the hydrogen atom:

Diagrams of the electronic structure of atoms show the distribution of electrons across electronic layers (energy levels).

Graphic electronic formula of the hydrogen atom (shows the distribution of electrons by energy levels and sublevels):

Graphic electronic formulas of atoms show the distribution of electrons not only among levels and sublevels, but also among orbitals.
In a helium atom, the first electron layer is complete - it has 2 electrons. Hydrogen and helium are s-elements; The s-orbital of these atoms is filled with electrons.

For all elements of the second period the first electronic layer is filled, and electrons fill the s and p orbitals of the second electron layer in accordance with the principle of least energy (first s and then p) and the Pauli and Hund rules.
In the neon atom, the second electron layer is complete - it has 8 electrons.
For atoms of elements of the third period, the first and second electronic layers are completed, so the third electronic layer is filled, in which electrons can occupy the 3s-, 3p- and 3d-sublevels.
The magnesium atom completes its 3s electron orbital. Na and Mg are s-elements.
In aluminum and subsequent elements, the 3p sublevel is filled with electrons.
Elements of the third period have unfilled 3d orbitals.
All elements from Al to Ar are p-elements. The s- and p-elements form the main subgroups in the Periodic Table.

Elements of the fourth - seventh periods
A fourth electron layer appears in potassium and calcium atoms, and the 4s sublevel is filled, since it has lower energy than the 3d sublevel.
K, Ca - s-elements included in the main subgroups. For atoms from Sc to Zn, the 3d sublevel is filled with electrons. These are 3d elements. They are included in secondary subgroups, their outermost electronic layer is filled, and they are classified as transition elements.
Pay attention to the structure of the electronic shells of chromium and copper atoms. In them, one electron “fails” from the 4s to the 3d sublevel, which is explained by the greater energy stability of the resulting electronic configurations 3d 5 and 3d 10:

In the zinc atom, the third electron layer is complete - all sublevels 3s, 3p and 3d are filled in it, with a total of 18 electrons. In the elements following zinc, the fourth electron layer, the 4p sublevel, continues to be filled.
Elements from Ga to Kr are p-elements.
The krypton atom has an outer layer (fourth) that is complete and has 8 electrons. But there can be a total of 32 electrons in the fourth electron layer; the krypton atom still has unfilled 4d and 4f sublevels. For elements of the fifth period, sublevels are being filled in the following order: 5s - 4d - 5p. And there are also exceptions related to “ failure» electrons, y 41 Nb, 42 Mo, 44 Ru, 45 Rh, 46 Pd, 47 Ag.
In the sixth and seventh periods, f-elements appear, i.e., elements in which the 4f- and 5f-sublevels of the third outside electronic layer are filled, respectively.
4f elements are called lanthanides.
5f elements are called actinides.
The order of filling electronic sublevels in the atoms of elements of the sixth period: 55 Cs and 56 Ba - 6s elements; 57 La … 6s 2 5d x - 5d element; 58 Ce - 71 Lu - 4f elements; 72 Hf - 80 Hg - 5d elements; 81 T1 - 86 Rn - 6d elements. But here, too, there are elements in which the order of filling the electron orbitals is “violated,” which, for example, is associated with the greater energy stability of half and fully filled f-sublevels, i.e. nf 7 and nf 14. Depending on which sublevel of the atom is filled with electrons last, all elements are divided into four electron families, or blocks:
- s-elements. The s-sublevel of the outer level of the atom is filled with electrons; s-elements include hydrogen, helium and elements of the main subgroups of groups I and II.
- p-elements. The p-sublevel of the outer level of the atom is filled with electrons; p-elements include elements of the main subgroups of groups III-VIII.
- d-elements. The d-sublevel of the pre-external level of the atom is filled with electrons; d-elements include elements of secondary subgroups of groups I-VIII, i.e. elements of plug-in decades of large periods located between s- and p-elements. They are also called transition elements.
- f-elements. The f-sublevel of the third outer level of the atom is filled with electrons; these include lanthanides and antinoids.

The Swiss physicist W. Pauli in 1925 established that in an atom in one orbital there can be no more than two electrons having opposite (antiparallel) spins (translated from English as “spindle”), i.e., having such properties that conditionally can be imagined as the rotation of an electron around its imaginary axis: clockwise or counterclockwise.

This principle is called Pauli principle. If there is one electron in the orbital, then it is called unpaired; if there are two, then these are paired electrons, i.e. electrons with opposite spins. The figure shows a diagram of the division of energy levels into sublevels and the order in which they are filled.


Very often, the structure of the electronic shells of atoms is depicted using energy or quantum cells - so-called graphical electronic formulas are written. For this notation, the following notation is used: each quantum cell is designated by a cell that corresponds to one orbital; Each electron is indicated by an arrow corresponding to the spin direction. When writing a graphical electronic formula, you should remember two rules: Pauli's principle and F. Hund's rule, according to which electrons occupy free cells first one at a time and have the same spin value, and only then pair, but the spins, according to the Pauli principle, will already be oppositely directed.
Hund's rule and Pauli's principle
Hund's rule- a rule of quantum chemistry that determines the order of filling the orbitals of a certain sublayer and is formulated as follows: the total value of the spin quantum number of electrons of a given sublayer must be maximum. Formulated by Friedrich Hund in 1925.
This means that in each of the orbitals of the sublayer, one electron is first filled, and only after the unfilled orbitals are exhausted, a second electron is added to this orbital. In this case, in one orbital there are two electrons with half-integer spins of the opposite sign, which pair (form a two-electron cloud) and, as a result, the total spin of the orbital becomes equal to zero.
Another wording: Lower in energy lies the atomic term for which two conditions are satisfied.
- Multiplicity is maximum
- When the multiplicities coincide, the total orbital momentum L is maximum.
Let us analyze this rule using the example of filling p-sublevel orbitals p-elements of the second period (that is, from boron to neon (in the diagram below, horizontal lines indicate orbitals, vertical arrows indicate electrons, and the direction of the arrow indicates the spin orientation).
Klechkovsky's rule
Klechkovsky's rule - as the total number of electrons in atoms increases (as the charges of their nuclei increase, or serial numbers chemical elements) atomic orbitals are populated in such a way that the appearance of electrons in an orbital with a higher energy depends only on the main quantum number n and does not depend on all other quantum numbers, including l. Physically, this means that in a hydrogen-like atom (in the absence of interelectron repulsion), the orbital energy of an electron is determined only by the spatial distance of the electron charge density from the nucleus and does not depend on the characteristics of its motion in the field of the nucleus.
The empirical Klechkovsky rule and the ordering scheme that follows from it are somewhat contradictory to the real energy sequence of atomic orbitals only in two similar cases: for atoms Cr, Cu, Nb, Mo, Ru, Rh, Pd, Ag, Pt, Au, there is a “failure” of an electron with s -sublevel of the outer layer is replaced by the d-sublevel of the previous layer, which leads to an energetically more stable state of the atom, namely: after filling orbital 6 with two electrons s
An atom is the smallest particle of a chemical substance that can retain its properties. The word "atom" comes from the ancient Greek "atomos", meaning "indivisible". Depending on how many and what particles are in an atom, a chemical element can be determined.
Briefly about the structure of the atom
How can you briefly list the basic information about is a particle with one nucleus, which is positively charged. Around this nucleus is a negatively charged cloud of electrons. Each atom in its normal state is neutral. The size of this particle can be entirely determined by the size of the electron cloud that surrounds the nucleus.
The nucleus itself, in turn, also consists of smaller particles - protons and neutrons. Protons are positively charged. Neutrons do not carry any charge. However, protons and neutrons are combined into one category and are called nucleons. If basic information about the structure of the atom is needed briefly, then this information can be limited to the listed data.

First information about the atom
The ancient Greeks suspected that matter could consist of small particles. They believed that everything that exists is made of atoms. However, such a view was purely philosophical in nature and cannot be interpreted scientifically.
The first to obtain basic information about the structure of the atom was an English scientist. It was this researcher who was able to discover that two chemical element can enter into different ratios, and each such combination will represent a new substance. For example, eight parts of the element oxygen give rise to carbon dioxide. Four parts oxygen is carbon monoxide.
In 1803, Dalton discovered the so-called law of multiple ratios in chemistry. Using indirect measurements (since not a single atom could then be examined under the microscopes of that time), Dalton made a conclusion about the relative weight of atoms.

Rutherford's research
Almost a century later, basic information about the structure of atoms was confirmed by another English chemist - the Scientist proposed a model of the electron shell of the smallest particles.
At that time, Rutherford's "Planetary Model of the Atom" was one of the the most important steps that chemistry could do. Basic information about the structure of the atom indicated that it was similar to solar system: electron particles rotate around the nucleus in strictly defined orbits, just as planets do.
Electronic shell of atoms and formulas of atoms of chemical elements
The electron shell of each atom contains exactly as many electrons as there are protons in its nucleus. This is why the atom is neutral. In 1913, another scientist obtained basic information about the structure of the atom. Niels Bohr's formula was similar to that obtained by Rutherford. According to his concept, electrons also revolve around the nucleus located at the center. Bohr refined Rutherford's theory and brought harmony to its facts.
Even then, formulas for some chemicals. For example, schematically the structure of the nitrogen atom is denoted as 1s 2 2s 2 2p 3, the structure of the sodium atom is expressed by the formula 1s 2 2s 2 2p 6 3s 1. Through these formulas you can see how many electrons move in each of the orbitals of a particular chemical substance.

Schrödinger model
However, later this atomic model also became outdated. Basic information about the structure of the atom, known to science today, largely became available thanks to the research of the Austrian physicist
He suggested new model its structure is wave. By this time, scientists had already proven that the electron is endowed not only with the nature of a particle, but also has the properties of a wave.
However, the Schrödinger and Rutherford model also has general provisions. Their theories are similar in that electrons exist at certain levels.
Such levels are also called electronic layers. Using the level number, the electron energy can be characterized. The higher the layer, the more energy it has. All levels are counted from bottom to top, so the level number corresponds to its energy. Each of the layers in the electron shell of an atom has its own sublevels. In this case, the first level may have one sublevel, the second - two, the third - three, and so on (see the above electronic formulas for nitrogen and sodium).

Even smaller particles
At the moment, of course, even smaller particles have been discovered than the electron, proton and neutron. It is known that the proton consists of quarks. There are even smaller particles of the universe - for example, the neutrino, which is a hundred times smaller in size than a quark and a billion times smaller than a proton.
A neutrino is such a small particle that it is 10 septillion times smaller than, for example, a tyrannosaurus rex. The tyrannosaurus itself is as many times smaller in size than the entire observable Universe.
Basic information about the structure of the atom: radioactivity
It was always known that not a single chemical reaction cannot transform one element into another. But in the process of radioactive radiation this happens spontaneously.
Radioactivity is the ability of atomic nuclei to transform into other nuclei - more stable ones. When people received basic information about the structure of atoms, isotopes, to a certain extent, could serve as the embodiment of the dreams of medieval alchemists.
As isotopes decay, radioactive radiation is emitted. This phenomenon was first discovered by Becquerel. Main view Radioactive radiation is alpha decay. When it occurs, an alpha particle is released. There is also beta decay, in which a beta particle is ejected from the nucleus of an atom.
Natural and artificial isotopes
Currently, about 40 natural isotopes are known. Most of them are located in three categories: uranium-radium, thorium and actinium. All these isotopes can be found in nature - in rocks, soil, air. But besides them, about a thousand artificially derived isotopes are also known, which are produced in nuclear reactors. Many of these isotopes are used in medicine, especially in diagnostics..

Proportions within an atom
If we imagine an atom whose dimensions are comparable to the dimensions of an international sports stadium, then we can visually obtain the following proportions. The electrons of an atom in such a “stadium” will be located at the very top of the stands. Each one will be smaller than the head of a pin. Then the core will be located in the center of this field, and its size will be no larger than the size of a pea.
Sometimes people ask what an atom actually looks like. In fact, it literally does not look like anything - not for the reason that the microscopes used in science are not good enough. The dimensions of an atom are in those areas where the concept of “visibility” simply does not exist.
Atoms are very small in size. But how small are these sizes really? The fact is that the smallest grain of salt, barely visible to the human eye, contains about one quintillion atoms.
If we imagine an atom of such a size that could fit in a human hand, then next to it there would be viruses 300 meters long. Bacteria would be 3 km long, and the thickness of a human hair would be 150 km. In a supine position, he would be able to go beyond the boundaries of the earth's atmosphere. And if such proportions were valid, then a human hair could reach the Moon in length. This is such a complex and interesting atom, which scientists continue to study to this day.
Atom- the smallest particle of a substance that is indivisible by chemical means. In the 20th century it was found out complex structure atom. Atoms are made up of positively charged kernels and a shell formed by negatively charged electrons. The total charge of a free atom is zero, since the charges of the nucleus and electron shell balance each other. In this case, the nuclear charge is equal to the number of the element in the periodic table ( atomic number) and is equal to the total number of electrons (electron charge is −1).
The atomic nucleus consists of positively charged protons and neutral particles - neutrons, having no charge. Generalized characteristics of elementary particles in an atom can be presented in the form of a table:
The number of protons is equal to the charge of the nucleus, therefore equal to the atomic number. To find the number of neutrons in an atom, you need to subtract the charge of the nucleus (the number of protons) from the atomic mass (consisting of the masses of protons and neutrons).
For example, in the sodium atom 23 Na the number of protons is p = 11, and the number of neutrons is n = 23 − 11 = 12
The number of neutrons in atoms of the same element can be different. Such atoms are called isotopes .
The electron shell of an atom also has a complex structure. Electrons are located in energy levels (electronic layers).
The level number characterizes the energy of the electron. This is due to the fact that elementary particles can transmit and receive energy not in arbitrarily small quantities, but in certain portions - quanta. The higher the level, the more energy the electron has. Since the lower the energy of the system, the more stable it is (compare the low stability of a stone on the top of a mountain, which has a large potential energy, and the stable position of the same stone below on the plain, when its energy is much lower), the levels with low electron energy are filled first and only then the high ones.
The maximum number of electrons that a level can accommodate can be calculated using the formula:
N = 2n 2, where N is the maximum number of electrons at the level,
n - level number.
Then for the first level N = 2 1 2 = 2,
for the second N = 2 2 2 = 8, etc.
Number of electrons per external level for elements of the main (A) subgroups is equal to the group number.
In most modern periodic tables, the arrangement of electrons by level is indicated in the cell with the element. Very important understand that the levels are readable from bottom to top, which corresponds to their energy. Therefore, the column of numbers in the cell with sodium:
1
8
2
at the 1st level - 2 electrons,
at the 2nd level - 8 electrons,
at the 3rd level - 1 electron
Be careful, this is a very common mistake!
The electron level distribution can be represented as a diagram:
11 Na)))
2 8 1
If the periodic table does not indicate the distribution of electrons by level, you can use:
- maximum number of electrons: at the 1st level no more than 2 e − ,
on the 2nd - 8 e − ,
at the external level - 8 e − ; - number of electrons in the outer level (for the first 20 elements coincides with the group number)
Then for sodium the line of reasoning will be as follows:
- The total number of electrons is 11, therefore, the first level is filled and contains 2 e − ;
- The third, outer level contains 1 e − (I group)
- The second level contains the remaining electrons: 11 − (2 + 1) = 8 (completely filled)
* A number of authors, in order to more clearly distinguish between a free atom and an atom as part of a compound, propose using the term “atom” only to designate a free (neutral) atom, and to designate all atoms, including those in compounds, propose the term “atomic particles”. Time will tell what the fate of these terms will be. From our point of view, an atom by definition is a particle, therefore, the expression “atomic particles” can be considered as a tautology (“oil”).
2. Task. Calculation of the amount of substance of one of the reaction products if the mass of the starting substance is known.
Example:
What amount of hydrogen substance will be released when zinc reacts with hydrochloric acid weighing 146 g?
Solution:
- We write the reaction equation: Zn + 2HCl = ZnCl 2 + H 2
- We find molar mass hydrochloric acid: M (HCl) = 1 + 35.5 = 36.5 (g/mol)
(the molar mass of each element, numerically equal to the relative atomic mass, is looked at in the periodic table under the sign of the element and rounded to whole numbers, except for chlorine, which is taken as 35.5) - Find the amount of hydrochloric acid: n (HCl) = m / M = 146 g / 36.5 g/mol = 4 mol
- We write down the available data above the reaction equation, and below the equation - the number of moles according to the equation (equal to the coefficient in front of the substance):
4 mol x mol
Zn + 2HCl = ZnCl 2 + H 2
2 mole 1 mole - Let's make a proportion:
4 mol - x mole
2 mol - 1 mol
(or with an explanation:
from 4 moles of hydrochloric acid you get x mole of hydrogen,
and from 2 moles - 1 mole) - We find x:
x= 4 mol 1 mol / 2 mol = 2 mol
Answer: 2 mol.