1 Despite the prefix, the kilogram is the basic unit of mass in the SI system. It is the kilogram, not the gram, that is used for calculations
Standard SI prefixes
| Name | Symbol | Factor |
| yocto- | y | 10 -24 |
| ceto- | z | 10 -21 |
| atto- | a | 10 -18 |
| femto- | f | 10 -15 |
| pico- | p | 10 -12 |
| nano- | n | 10 -9 |
| micro- | µ | 10 -6 |
| Milli- | m | 10 -3 |
| centi- | c | 10 -2 |
| deci- | d | 10 -1 |
| deca- | da | 10 1 |
| hecto- | h | 10 2 |
| kilo- | k | 10 3 |
| mega- | M | 10 6 |
| giga- | G | 10 9 |
| tera- | T | 10 12 |
| peta- | P | 10 15 |
| exa- | E | 10 18 |
| zetta- | Z | 10 21 |
| yotta- | Y | 10 24 |
Derived units
Derived units can be expressed in terms of base units using the mathematical operations of multiplication and division. Some of the derived units are given their own names for convenience; such units can also be used in mathematical expressions to form other derived units.
The mathematical expression for the derived unit of measurement follows from physical law, with the help of which this unit of measurement is defined or the definition of the physical quantity for which it is introduced. For example, speed is the distance a body travels per unit time. Accordingly, the unit of measurement for speed is m/s (meter per second).
Often the same unit of measurement can be written in different ways, using a different set of base and derived units (see, for example, the last column in the table ). However, in practice, established (or simply generally accepted) expressions are used that best reflect physical meaning measured quantity. For example, to write the value of a moment of force, you should use N×m, and you should not use m×N or J.
| Magnitude | Unit | Designation | Expression | ||
|---|---|---|---|---|---|
| Russian name | international name | Russian | international | ||
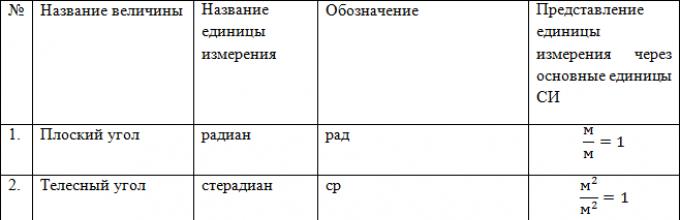
| Flat angle | radian | radian | glad | rad | m×m -1 = 1 |
| Solid angle | steradian | steradian | Wed | sr | m 2 ×m -2 = 1 |
| Temperature in Celsius | degrees Celsius | °C | degree Celsius | °C | K |
| Frequency | hertz | hertz | Hz | Hz | s -1 |
| Force | newton | newton | N | N | kg×m/s 2 |
| Energy | joule | joule | J | J | N×m = kg×m 2 /s 2 |
| Power | watt | watt | W | W | J/s = kg × m 2 / s 3 |
| Pressure | pascal | pascal | Pa | Pa | N/m 2 = kg? m -1 ? s 2 |
| Light flow | lumen | lumen | lm | lm | kd×sr |
| Illumination | luxury | lux | OK | lx | lm/m 2 = cd×sr×m -2 |
| Electric charge | pendant | coulomb | Cl | C | А×с |
| Potential difference | volt | volt | IN | V | J/C = kg×m 2 ×s -3 ×A -1 |
| Resistance | ohm | ohm | Ohm | Ω | V/A = kg×m 2 ×s -3 ×A -2 |
| Capacity | farad | farad | F | F | C/V = kg -1 ×m -2 ×s 4 ×A 2 |
| Magnetic flux | weber | weber | Wb | Wb | kg×m 2 ×s -2 ×A -1 |
| Magnetic induction | tesla | tesla | Tl | T | Wb/m 2 = kg × s -2 × A -1 |
| Inductance | Henry | Henry | Gn | H | kg×m 2 ×s -2 ×A -2 |
| Electrical conductivity | Siemens | siemens | Cm | S | Ohm -1 = kg -1 ×m -2 ×s 3 A 2 |
| Radioactivity | becquerel | becquerel | Bk | Bq | s -1 |
| Absorbed dose ionizing radiation | Gray | gray | Gr | Gy | J/kg = m 2 / s 2 |
| Effective dose of ionizing radiation | sievert | sievert | Sv | Sv | J/kg = m 2 / s 2 |
| Catalyst activity | rolled | catal | cat | kat | mol×s -1 |
Units not included in the SI System
Some units of measurement not included in the SI System are, by decision of the General Conference on Weights and Measures, “allowed for use in conjunction with SI.”
| Unit | International name | Designation | Value in SI units | |
|---|---|---|---|---|
| Russian | international | |||
| minute | minute | min | min | 60 s |
| hour | hour | h | h | 60 min = 3600 s |
| day | day | days | d | 24 h = 86,400 s |
| degree | degree | ° | ° | (P/180) glad |
| arcminute | minute | ′ | ′ | (1/60)° = (P/10,800) |
| arcsecond | second | ″ | ″ | (1/60)′ = (P/648,000) |
| liter | liter (liter) | l | l, L | 1 dm 3 |
| ton | tons | T | t | 1000 kg |
| neper | neper | Np | Np | |
| white | bel | B | B | |
| electron-volt | electronvolt | eV | eV | 10 -19 J |
| atomic mass unit | unified atomic mass unit | A. eat. | u | =1.49597870691 -27 kg |
| astronomical unit | astronomical unit | A. e. | ua | 10 11 m |
| nautical mile | nautical mile | mile | 1852 m (exactly) | |
| node | knot | bonds | 1 nautical mile per hour = (1852/3600) m/s | |
| ar | are | A | a | 10 2 m 2 |
| hectare | hectare | ha | ha | 10 4 m 2 |
| bar | bar | bar | bar | 10 5 Pa |
| angstrom | ångström | Å | Å | 10 -10 m |
| barn | barn | b | b | 10 -28 m 2 |
SI system(Le Système International d'Unités - The International System) was adopted by the XI General Conference on Weights and Measures, some subsequent conferences made a number of changes to the SI.
The SI defines seven basic and derived units physical quantities(hereinafter referred to as units), as well as a set of consoles. Standard abbreviations for units and rules for recording derived units have been established.
Basic units: kilogram, meter, second, ampere, kelvin, mole and candela. Within the SI framework, these units are considered to have independent dimensions, that is, none of the basic units can be derived from the others.
Derived units are obtained from the basic ones using algebraic operations such as multiplication and division. Some of the SI derived units are given their own names, such as the radian.
Prefix and can be used before unit names; they mean that a unit must be multiplied or divided by a certain integer, a power of 10. For example, the prefix “kilo” means multiplied by 1000 (kilometer = 1000 meters). SI prefixes are also called decimal prefixes.
Table 1. Basic SI units
|
Magnitude |
Unit |
Designation |
||
|
Russian name |
international name |
international |
||
|
kilogram |
||||
|
Current strength |
||||
|
Thermodynamic temperature |
||||
|
The power of light |
||||
|
Quantity of substance |
||||
Table 2. Derived SI units
|
Magnitude |
Unit |
Designation |
||
|
Russian name |
international name |
international |
||
|
Flat angle |
||||
|
Solid angle |
steradian |
|||
|
Celsius temperature¹ |
degrees Celsius |
|||
|
Power |
||||
|
Pressure |
||||
|
Light flow |
||||
|
Illumination |
||||
|
Electric charge |
||||
|
Potential difference |
||||
|
Resistance |
||||
|
Electrical capacity |
||||
|
Magnetic flux |
||||
|
Magnetic induction |
||||
|
Inductance |
||||
|
Electrical conductivity |
||||
|
Activity (radioactive source) |
becquerel |
|||
|
Absorbed dose of ionizing radiation |
||||
|
Effective dose of ionizing radiation |
||||
|
Catalyst activity |
||||
Source: http://ru.wikipedia.org/wiki/%D0%A1%D0%98
The Kelvin and Celsius scales are related as follows: °C = K - 273.15
Multiples of units- units that are an integer number of times greater than the basic unit of measurement of some physical quantity. The International System of Units (SI) recommends the following decimal prefixes to represent multiple units:
Table 3. Multiples
|
Multiplicity |
Console |
Designation |
||
|
international |
international |
|||
The variety of individual units (force, for example, could be expressed in kg, pounds, etc.) and systems of units created great difficulties in the worldwide exchange of scientific and economic achievements. Therefore, back in the 19th century, there was a need to create a unified international system that would include units of measurement of quantities used in all branches of physics. However, agreement to introduce such a system was adopted only in 1960.
International system of units is a correctly constructed and interconnected set of physical quantities. It was adopted in October 1960 at the 11th General Conference on Weights and Measures. The abbreviated name of the system is SI. In Russian transcription - SI. (international system).
In the USSR, GOST 9867-61 was introduced in 1961, which established the preferable use of this system in all areas of science, technology, and teaching. Currently, the current GOST 8.417-81 “GSI. Units of physical quantities". This standard establishes the units of physical quantities used in the USSR, their names, designations and rules of application. It was developed in full accordance with the SI system and ST SEV 1052-78.
The C system consists of seven basic units, two additional units and a number of derivatives. In addition to SI units, the use of submultiples and multiples is allowed, obtained by multiplying the original values by 10 n, where n = 18, 15, 12, ... -12, -15, -18. The names of multiple and submultiple units are formed by adding the corresponding decimal prefixes:
exa (E) = 10 18; peta (P) = 10 15 ; tera (T) = 10 12 ; giga (G) = 10 9 ; mega (M) = 10 6 ;
miles (m) = 10 –3 ; micro (μ) = 10 –6; nano(n) = 10 –9; pico(p) = 10 –12;
femto (f) = 10 –15; atto(a) = 10 –18;
GOST 8.417-81 allows the use, in addition to the specified units, of a number of non-systemic units, as well as units temporarily permitted for use until the relevant international decisions are adopted.
The first group includes: ton, day, hour, minute, year, liter, light year, volt-ampere.
The second group includes: nautical mile, carat, knot, rpm.
1.4.4 Basic units of SI.
Unit of length – meter (m)
A meter is equal to 1650763.73 wavelengths in vacuum of radiation corresponding to the transition between the 2p 10 and 5d 5 levels of the krypton-86 atom.
The International Bureau of Weights and Measures and large national metrology laboratories have created installations for reproducing the meter in light wavelengths.
The unit of mass is kilogram (kg).
Mass is a measure of the inertia of bodies and their gravitational properties. A kilogram is equal to the mass of the international prototype of the kilogram.
The state primary standard of the SI kilogram is intended for reproduction, storage and transfer of the unit of mass to working standards.
The standard includes:
A copy of the international prototype of the kilogram - platinum-iridium prototype No. 12, which is a weight in the form of a cylinder with a diameter and height of 39 mm.
Equal-arm prismatic scales No. 1 for 1 kg with remote control from Ruphert (1895) and No. 2 manufactured at VNIIM in 1966.
Once every 10 years, the state standard is compared with a copy standard. Over 90 years, the mass of the state standard has increased by 0.02 mg due to dust, adsorption and corrosion.
Now mass is the only unit quantity that is determined through a real standard. This definition has a number of disadvantages - change in the mass of the standard over time, irreproducibility of the standard. Research is underway to express a unit of mass through natural constants, for example through the mass of a proton. It is also planned to develop a standard using a certain number of Si-28 silicon atoms. To solve this problem, first of all, the accuracy of measuring Avogadro's number must be increased.
The unit of time is second (s).
Time is one of the central concepts of our worldview, one of the most important factors in the life and activities of people. It is measured using stable periodic processes - the annual rotation of the Earth around the Sun, daily - the rotation of the Earth around its axis, and various oscillatory processes. The definition of the unit of time, the second, has changed several times in accordance with the development of science and requirements for measurement accuracy. The current definition is:
A second is equal to 9192631770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium 133 atom.
Currently, a beam standard of time, frequency and length has been created, used by the time and frequency service. Radio signals allow the transmission of a unit of time, so it is widely available. The standard second error is 1·10 -19 s.
Unit of force electric current– ampere (A)
An ampere is equal to the strength of an unchanging current, which, when passing through two parallel and straight conductors of infinite length and negligibly small area cross section, located in a vacuum at a distance of 1 meter from each other, would cause an interaction force equal to 2·10 -7 N on each section of a conductor 1 meter long.
The error of the ampere standard is 4·10 -6 A. This unit is reproduced using the so-called current scales, which are accepted as the ampere standard. It is planned to use 1 volt as the main unit, since its reproduction error is 5·10 -8 V.
Unit of thermodynamic temperature – Kelvin (K)
Temperature is a value that characterizes the degree of heating of a body.
Since the invention of the Thermometer by Galileo, temperature measurement has been based on the use of one or another thermometric substance that changes its volume or pressure with a change in temperature.
All known temperature scales (Fahrenheit, Celsius, Kelvin) are based on some reference points to which different numerical values are assigned.
Kelvin and, independently of him, Mendeleev expressed considerations about the advisability of constructing a temperature scale based on one reference point, which was taken as the “triple point of water,” which is the equilibrium point of water in the solid, liquid and gaseous phases. It can currently be reproduced in special vessels with an error of no more than 0.0001 degrees Celsius. The lower limit of the temperature range is the absolute zero point. If this interval is divided into 273.16 parts, you get a unit of measurement called Kelvin.
Kelvin is 1/273.16 part of the thermodynamic temperature of the triple point of water.
The symbol T is used to denote temperature expressed in Kelvin, and t in degrees Celsius. The transition is made according to the formula: T=t+ 273.16. A degree Celsius is equal to one Kelvin (both units are eligible for use).
The unit of luminous intensity is candela (cd)
Luminous intensity is a quantity that characterizes the glow of a source in a certain direction, equal to the ratio of the luminous flux to the small solid angle in which it propagates.
The candela is equal to the luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540·10 12 Hz, the luminous energy intensity of which in that direction is 1/683 (W/sr) (Watts per steradian).
The error in reproducing a unit with a standard is 1·10 -3 cd.
The unit of quantity of a substance is the mole.
A mole is equal to the amount of substance in a system containing the same number of structural elements as there are atoms in C12 carbon weighing 0.012 kg.
When using mole structural elements must be specified and may be atoms, molecules, ions, electrons, or specified groups of particles.
Additional SI units
The international system includes two additional units - for measuring plane and solid angles. They cannot be basic, since they are dimensionless quantities. Assigning an independent dimension to an angle would lead to the need to change the mechanics equations related to rotational and curvilinear motion. At the same time, they are not derivatives, since they do not depend on the choice of basic units. Therefore, these units are included in the SI as additional ones necessary for the formation of some derived units - angular velocity, angular acceleration, etc.
The unit of plane angle is radian (rad)
A radian is equal to the angle between two radii of a circle, the length of the arc between which is equal to the radius.
The state primary standard of the radian consists of a 36-sided prism and a standard goniometric autocollimation installation with a division value of the reading devices of 0.01’’. The reproduction of the plane angle unit is carried out by the calibration method, based on the fact that the sum of all central angles of a polyhedral prism is equal to 2π rad.
The unit of solid angle is steradian (sr)
The steradian is equal to the solid angle with its vertex at the center of the sphere, cutting out the area on the surface of the sphere, equal to the area square with a side equal to the radius of the sphere.
The solid angle is measured by determining the plane angles at the vertex of the cone. The solid angle 1ср corresponds to a flat angle 65 0 32’. For recalculation use the formula:
where Ω is the solid angle in sr; α is the plane angle at the vertex in degrees.
The solid angle π corresponds to a plane angle of 120 0, and the solid angle 2π corresponds to a plane angle of 180 0.
Usually angles are measured in degrees - this is more convenient.
Advantages of SI
It is universal, that is, it covers all measurement areas. With its implementation, you can abandon all other unit systems.
It is coherent, that is, a system in which the derived units of all quantities are obtained using equations with numerical coefficients equal to the dimensionless unit (the system is coherent and consistent).
The units in the system are unified (instead of a number of units of energy and work: kilogram-force-meter, erg, calorie, kilowatt-hour, electron-volt, etc. - one unit for measuring work and all types of energy - joule).
There is a clear distinction between units of mass and force (kg and N).
Disadvantages of SI
Not all units have a size convenient for practical use: the pressure unit Pa is a very small value; unit of electrical capacitance F is a very large value.
Inconvenience of measuring angles in radians (degrees are easier to perceive)
Many derived quantities do not yet have their own names.
Thus, the adoption of SI is the next and very important step in the development of metrology, a step forward in improving systems of units of physical quantities.
In 1875, the International Bureau of Weights and Measures was founded by the Metric Conference; its purpose was to create unified system measurements that would find application throughout the world. It was decided to take as a basis the metric system, which appeared during the French Revolution and was based on the meter and kilogram. Later, the standards of the meter and kilogram were approved. Over time, the system of units of measurement has evolved and currently has seven basic units of measurement. In 1960 this system of units received modern name International System of Units (SI System) (Systeme Internatinal d "Unites (SI)). The SI system is not static; it is developing in accordance with the requirements that are currently imposed on measurements in science and technology.
Basic units of measurement of the International System of Units
The definition of all auxiliary units in the SI system is based on seven basic units of measurement. The main physical quantities in the International System of Units (SI) are: length ($l$); mass ($m$); time ($t$); electric current ($I$); Kelvin temperature (thermodynamic temperature) ($T$); amount of substance ($\nu$); luminous intensity ($I_v$).
The basic units in the SI system are the units of the above-mentioned quantities:
\[\left=m;;\ \left=kg;;\ \left=s;\ \left=A;;\ \left=K;;\ \ \left[\nu \right]=mol;;\ \left=cd\ (candela).\]
Standards of basic units of measurement in SI
Let us present the definitions of the standards of basic units of measurement as done in the SI system.
Meter (m) is the length of the path that light travels in a vacuum in a time equal to $\frac(1)(299792458)$ s.
Standard mass for SI is a weight in the shape of a straight cylinder, the height and diameter of which is 39 mm, consisting of an alloy of platinum and iridium weighing 1 kg.
One second (s) called a time interval that is equal to 9192631779 periods of radiation, which corresponds to the transition between two hyperfine levels of the ground state of the cesium atom (133).
One ampere (A)- this is the current strength passing in two straight infinitely thin and long conductors located at a distance of 1 meter, located in a vacuum, generating the Ampere force (the force of interaction of conductors) equal to $2\cdot (10)^(-7)N$ for each meter of conductor .
One kelvin (K)- this is the thermodynamic temperature equal to $\frac(1)(273.16)$ part of the triple point temperature of water.
One mole (mole)- this is the amount of a substance that has the same number of atoms as there are in 0.012 kg of carbon (12).
One candela (cd) equal to the intensity of light emitted by a monochromatic source with a frequency of $540\cdot (10)^(12)$Hz with an energy force in the direction of radiation $\frac(1)(683)\frac(W)(avg).$
Science is developing, measuring technology is being improved, and definitions of units of measurement are being revised. The higher the measurement accuracy, the greater the requirements for determining units of measurement.
SI derived quantities
All other quantities are considered in the SI system as derivatives of the basic ones. The units of measurement of derived quantities are defined as the result of the product (taking into account the degree) of the basic ones. Let us give examples of derived quantities and their units in the SI system.
The SI system also has dimensionless quantities, for example, reflection coefficient or relative dielectric constant. These quantities have dimension one.
The SI system includes derived units with special names. These names are compact forms of representing combinations of basic quantities. Let us give examples of SI units that have their own names (Table 2).

Each SI quantity has only one unit, but the same unit can be used for different quantities. Joule is a unit of measurement for the amount of heat and work.
SI system, units of measurement multiples and submultiples
The International System of Units has a set of prefixes for units of measurement that are used if the numerical values of the quantities in question are significantly greater or less than the system unit that is used without the prefix. These prefixes are used with any units of measurement; in the SI system they are decimal.
Let us give examples of such prefixes (Table 3).

When writing, the prefix and the name of the unit are written together, so that the prefix and the unit of measurement form a single symbol.
Note that the unit of mass in the SI system (kilogram) has historically already had a prefix. Decimal multiples and submultiples of the kilogram are obtained by connecting the prefix to the gram.
Non-system units
The SI system is universal and convenient in international communication. Almost all units that are not included in the SI system can be defined using SI terms. The use of the SI system is preferred in science education. However, there are some quantities that are not included in the SI, but are widely used. Thus, units of time such as minute, hour, day are part of culture. Some units are used for historical reasons. When using units that do not belong to the SI system, it is necessary to indicate how they are converted to SI units. An example of units is given in Table 4.
System of units of physical quantities, a modern version of the metric system. SI is the most widely used system of units in the world, as in Everyday life, and in science and technology. SI is now accepted as the primary system of units by most countries in the world and is almost always used in engineering, even in countries where traditional units are used in everyday life. In these few countries (eg the US), the definitions of traditional units have been modified to relate them by fixed factors to the corresponding SI units.
The SI was adopted by the XI General Conference on Weights and Measures in 1960, and several subsequent conferences made a number of changes to the SI.
In 1971, the XIV General Conference on Weights and Measures amended the SI, adding, in particular, a unit of quantity of a substance (mole).
In 1979, the XVI General Conference on Weights and Measures adopted a new definition of the candela that is still in effect today.
In 1983 year XVII The General Conference on Weights and Measures adopted a new definition of the meter that is still in effect today.
SI defines seven basic and derived units of physical quantities (hereinafter referred to as units), as well as a set of prefixes. Standard abbreviations for units and rules for recording derived units have been established.
Basic units: kilogram, meter, second, ampere, kelvin, mole and candela. Within the SI framework, these units are considered to have independent dimensions, that is, none of the basic units can be derived from the others.
Derived units are obtained from basic units using algebraic operations such as multiplication and division. Some of the SI derived units are given their own names, such as the radian.
Prefixes can be used before unit names; they mean that a unit must be multiplied or divided by a certain integer, a power of 10. For example, the prefix “kilo” means multiplied by 1000 (kilometer = 1000 meters). SI prefixes are also called decimal prefixes.
Many non-systemic units, such as, for example, ton, hour, liter and electron-volt are not included in the SI, but they are “allowed for use on an equal basis with SI units.”
Seven basic units and the dependence of their definitions
Basic SI units
|
Unit |
Designation |
Magnitude |
Definition |
Historical Origins/Rationale |
|
A meter is the length of the path traveled by light in a vacuum in a time interval of 1/299,792,458 seconds. |
1⁄10,000,000 of the distance from the Earth's equator to the north pole on the meridian of Paris. |
|||
|
Kilogram |
The kilogram is a unit of mass, equal to mass international prototype of the kilogram. |
The mass of one cubic decimeter (liter) of pure water at a temperature of 4 C and standard atmospheric pressure at sea level. |
||
|
A second is a time equal to 9,192,631,770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium-133 atom. |
The day is divided into 24 hours, each hour is divided into 60 minutes, each minute is divided into 60 seconds. |
|||
|
Electric current strength |
An ampere is the force of an unchanging current which, when passing through two parallel straight conductors of infinite length and negligibly small circular cross-sectional area, located in a vacuum at a distance of 1 m from each other, would cause on each section of the conductor 1 m long an interaction force equal to 2 ·10 −7 newtons. |
|||
|
Thermodynamic Temperature |
Kelvin is a unit of thermodynamic temperature equal to 1/273.16 of the thermodynamic temperature of the triple point of water. |
The Kelvin scale uses the same increments as the Celsius scale, but 0 Kelvin is the temperature of absolute zero, not the melting point of ice. According to modern definition The zero of the Celsius scale is set in such a way that the temperature of the triple point of water is 0.01 C. As a result, the Celsius and Kelvin scales are shifted by 273.15 ° C = K - 273.15. |
||
|
Quantity of substance |
A mole is the amount of substance in a system containing the same number of structural elements as there are atoms in carbon-12 weighing 0.012 kg. When using a mole, the structural elements must be specified and can be atoms, molecules, ions, electrons and other particles or specified groups of particles. |
|||
|
The power of light |
Candela is the luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540·10 12 hertz, the energetic luminous intensity of which in this direction is (1/683) W/sr. |
|
Magnitude |
Unit |
|||||
|
Name |
Dimension |
Name |
Designation |
|||
|
Russian |
French/English |
Russian |
international |
|||
|
kilogram |
kilogramme/kilogram |
|||||
|
Electric current strength |
||||||
|
Thermodynamic temperature |
||||||
|
Quantity of substance |
mole |
|||||
|
The power of light |
||||||
Derived units with their own names
|
Magnitude |
Unit |
Designation |
Expression |
||
|
Russian name |
French/English title |
Russian |
international |
||
|
Flat angle |
|||||
|
Solid angle |
steradian |
m 2 m −2 = 1 |
|||
|
Temperature in Celsius |
degrees Celsius |
degree Celsius/degree Celsius |
|||
|
kg m s −2 |
|||||
|
N m = kg m 2 s −2 |
|||||
|
Power |
J/s = kg m 2 s −3 |
||||
|
Pressure |
N/m 2 = kg m −1 s −2 |
||||
|
Light flow |
|||||
|
Illumination |
lm/m² = cd·sr/m² |
||||
|
Electric charge |
|||||
|
Potential difference |
J/C = kg m 2 s −3 A −1 |
||||
|
Resistance |
V/A = kg m 2 s −3 A −2 |
||||
|
Electrical capacity |
C/V = s 4 A 2 kg −1 m −2 |
||||
|
Magnetic flux |
kg m 2 s −2 A −1 |
||||
|
Magnetic induction |
Wb/m 2 = kg s −2 A −1 |
||||
|
Inductance |
kg m 2 s −2 A −2 |
||||
|
Electrical conductivity |
Ohm −1 = s 3 A 2 kg −1 m −2 |
||||
|
Radioactive source activity |
becquerel |
||||
|
Absorbed dose of ionizing radiation |
J/kg = m²/s² |
||||
|
Effective dose of ionizing radiation |
J/kg = m²/s² |
||||
|
Catalyst activity |
|||||
Units not included in the SI, but by decision of the General Conference on Weights and Measures, are “allowed for use in conjunction with the SI.”
|
Unit |
French/English title |
Designation |
Value in SI units |
|
|
Russian |
international |
|||
|
60 min = 3600 s |
||||
|
24 h = 86,400 s |
||||
|
arcminute |
(1/60)° = (π/10,800) |
|||
|
arcsecond |
(1/60)′ = (π/648,000) |
|||
|
dimensionless |
||||
|
dimensionless |
||||
|
electron-volt |
≈1.602 177 33·10 −19 J |
|||
|
atomic mass unit, dalton |
unité de masse atomique unifiée, dalton/unified atomic mass unit, dalton |
≈1,660 540 2·10 −27 kg |
||
|
astronomical unit |
unité astronomique/astronomical unit |
149 597 870 700 m (exactly) |
||
|
nautical mile |
mille marin/nautical mile |
1852 m (exactly) |
||
|
1 nautical mile per hour = (1852/3600) m/s |
||||
|
angstrom |
||||
Rules for writing unit symbols
Unit designations are printed in straight font; a dot is not placed after the designation as an abbreviation sign.
Designations are placed after the numerical values of quantities separated by a space; transfer to another line is not allowed. Exceptions are notations in the form of a sign above a line; they are not preceded by a space. Examples: 10 m/s, 15°.
If the numeric value is a fraction with a slash, it is enclosed in parentheses, for example: (1/60) s −1.
When indicating the values of quantities with maximum deviations, they are enclosed in brackets or a unit designation is placed behind the numerical value of the quantity and its maximum deviation: (100.0 ± 0.1) kg, 50 g ± 1 g.
Unit designations included in the product are separated by dots on midline(N·m, Pa·s), it is not allowed to use the symbol “×” for this purpose. In typewritten texts, it is allowed not to raise the period or to separate symbols with spaces if this does not cause misunderstandings.
You can use a horizontal bar or a slash (only one) as a division sign in notation. When using a slash, if the denominator contains a product of units, it is enclosed in parentheses. Correct: W/(m·K), incorrect: W/m/K, W/m·K.
It is allowed to use unit designations in the form of a product of unit designations raised to powers (positive and negative): W m −2 K −1 , A m². When using negative powers, you must not use a horizontal bar or a slash (divide sign).
It is allowed to use combinations of special characters with letter designations, for example: °/s (degrees per second).
It is not allowed to combine designations and full names of units. Incorrect: km/h, correct: km/h.
Unit designations derived from surnames are written with capital letters, including those with SI prefixes, for example: ampere - A, megapascal - MPa, kilonewton - kN, gigahertz - GHz.