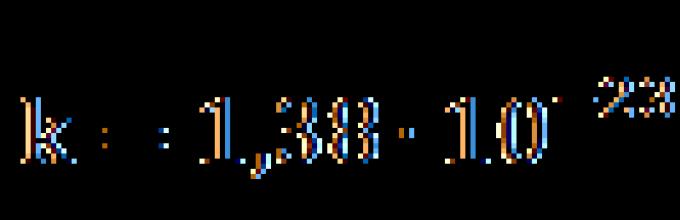born March 29, 1561 Italian doctor Santorio is one of the inventors of the first mercury thermometer, a device that was an innovation for that time and which no one can do without today.
Santorio was not only a doctor, but also an anatomist and physiologist. He worked in Poland, Hungary and Croatia, actively studied the breathing process, “invisible evaporations” from the surface of the skin, and conducted research in the field of human metabolism. Santorio conducted experiments on himself and, studying the characteristics of the human body, created many measuring instruments - a device for measuring the force of pulsation of arteries, scales for monitoring changes in human weight, and the first mercury thermometer.
Three inventors
It is quite difficult to say today who exactly created the thermometer. The invention of the thermometer is attributed to many scientists at once - Galileo, Santorio, Lord Bacon, Robert Fludd, Scarpi, Cornelius Drebbel, Porte and Salomon de Caus. This is due to the fact that many scientists simultaneously worked on creating a device that would help measure the temperature of air, soil, water, and humans.
There is no description of this device in Galileo's own writings, but his students testified that in 1597 he created a thermoscope - an apparatus for raising water using heat. The thermoscope was a small glass ball with a glass tube soldered to it. The difference between a thermoscope and a modern thermometer is that in Galileo's invention, instead of mercury, air expanded. Also, it could only be used to judge the relative degree of heating or cooling of the body, since it did not yet have a scale.
Santorio from the University of Padua created his own device with which it was possible to measure the temperature of the human body, but the device was so bulky that it was installed in the courtyard of a house. Santorio's invention had the shape of a ball and an oblong winding tube on which divisions were drawn; the free end of the tube was filled with a tinted liquid. His invention dates back to 1626.
In 1657, Florentine scientists improved the Galileo thermoscope, in particular by equipping the device with a bead scale.
Later, scientists tried to improve the device, but all thermometers were air, and their readings depended not only on changes in body temperature, but also on atmospheric pressure.
The first liquid thermometers were described in 1667, but they burst if the water froze, so they began to use wine alcohol to create them. The invention of a thermometer, the data of which would not be determined by changes in atmospheric pressure, occurred thanks to the experiments of the physicist Evangelista Torricelli, a student of Galileo. As a result, the thermometer was filled with mercury, turned upside down, colored alcohol was added to the ball and the upper end of the tube was sealed.
Single scale and mercury
For a long time, scientists could not find starting points, the distance between which could be divided evenly.
The initial data for the scale were the thawing points of ice and melted butter, the boiling point of water, and some abstract concepts like “a significant degree of cold.”
A thermometer of a modern form, most suitable for household use, with an accurate measurement scale was created by the German physicist Gabriel Fahrenheit. He described his method for creating a thermometer in 1723. Initially, Fahrenheit created two alcohol thermometers, but then the physicist decided to use mercury in the thermometer. The Fahrenheit scale was based on three established points:
the first point was equal to zero degrees - this is the temperature of the composition of water, ice and ammonia;
the second, designated 32 degrees, is the temperature of the mixture of water and ice;
the third, the boiling point of water, was 212 degrees.
The scale was later named after its creator.
Reference
Today, the most common is the Celsius scale, the Fahrenheit scale is still used in the USA and England, and the Kelvin scale is used in scientific research.
But it was the Swedish astronomer, geologist and meteorologist Anders Celsius who finally established both constant points - melting ice and boiling water - in 1742. He divided the distance between points into 100 intervals, with the number 100 marking the melting point of ice, and 0 the boiling point of water.
Today, the Celsius scale is used inverted, that is, the melting point of ice is taken as 0°, and the boiling point of water as 100°.
According to one version, the scale was “turned over” by his contemporaries and compatriots, the botanist Carl Linnaeus and the astronomer Morten Stremer, after the death of Celsius, but according to another, Celsius himself turned over his scale on Stremer’s advice.
In 1848, the English physicist William Thomson (Lord Kelvin) proved the possibility of creating an absolute temperature scale, where the reference point is the value of absolute zero: -273.15 ° C - at this temperature further cooling of bodies is no longer possible.
Already in mid-18th century centuries, thermometers became an item of trade, and they were made by artisans, but thermometers came into medicine much later, in the middle of the 19th century.
Modern thermometers
If in the 18th century there was a “boom” of discoveries in the field of temperature measurement systems, today work is increasingly being carried out to create methods for measuring temperature.
The scope of application of thermometers is extremely wide and is of particular importance for modern life person. A thermometer outside the window reports the temperature outside, a thermometer in the refrigerator helps control the quality of food storage, a thermometer in the oven allows you to maintain the temperature when baking, and a thermometer measures body temperature and helps assess the causes of poor health.
A thermometer is the most common type of thermometer, and it is the one that can be found in every home. However, mercury thermometers, which were once a brilliant discovery by scientists, are now gradually becoming a thing of the past as unsafe. Mercury thermometers contain 2 grams of mercury and have the highest accuracy in determining temperature, but you not only need to handle them correctly, but also know what to do if the thermometer suddenly breaks.
Mercury thermometers are being replaced by electronic or digital thermometers, which operate on the basis of a built-in metal sensor. There are also special thermal strips and infrared thermometers.
If mechanics in the 18th century became a mature, well-defined field of natural science, then the science of heat essentially took only its first steps. Of course, a new approach to the study of thermal phenomena emerged back in the 17th century. Galileo's thermoscope and the subsequent thermometers of the Florentine academicians, Guericke, and Newton prepared the ground on which thermometry grew already in the first quarter of the new century. Thermometers of Fahrenheit, Delisle, Lomonosov, Reaumur and Celsius, differing from each other in design features, at the same time determined the type of thermometer with two constant points, which is still accepted today.
Back in 1703, the Parisian academician Amonton (1663-1705) designed a gas thermometer in which the temperature was determined using a manometric tube connected to a gas reservoir of constant volume. Interesting in theoretically the device, a prototype of modern hydrogen thermometers, was inconvenient for practical purposes. The Danzig (Gdansk) glassblower Fahrenheit (1686-1736) had been producing alcohol thermometers with constant points since 1709. In 1714 he began producing mercury thermometers. Fahrenheit took the freezing point of water as 32°, the boiling point of water as 212°. Fahrenheit was considered to be the freezing point of a mixture of water, ice and ammonia or table salt. He named the boiling point of water only in 1724 in a printed publication. Whether he used it before is unknown.
The French zoologist and metallurgist Reaumur (1683-1757) proposed a thermometer with a constant zero point, for which he took the freezing point of water. Using an 80% alcohol solution as a thermometric body, and in the final version mercury, he took the boiling point of water as the second constant point, designating it as the number 80. Reaumur described his thermometer in articles published in the journal of the Paris Academy of Sciences in 1730, 1731 gg.
The test of Reaumur's thermometer was carried out by the Swedish astronomer Celsius (1701-1744), who described his experiments in 1742. “These experiments,” he wrote, “I repeated for two years, in all winter months, in different weather and various changes in the state of the barometer and always found exactly the same point on the thermometer. I not only placed the thermometer in melting ice, but also, in extreme cold, brought snow into my room on the fire until it began to melt. I also placed a cauldron with melting snow together with a thermometer in a heating stove and always found that the thermometer showed the same point, if only the snow lay tightly around the thermometer ball. After carefully checking the constancy of the melting point of ice, Celsius examined the boiling point of water and found that it depended on pressure. As a result of the research, a new thermometer appeared, now known as the Celsius thermometer. Celsius took the melting point of ice as 100, the boiling point of water at a pressure of 25 inches 3 lines of mercury as 0. The famous Swedish botanist Carl Linnaeus (1707-1788) used a thermometer with rearranged values of constant points. O meant the melting point of ice, 100 meant the boiling point of water. Thus, the modern Celsius scale is essentially the Linnaean scale.
At the St. Petersburg Academy of Sciences, Academician Delisle proposed a scale in which the melting point of ice was taken as 150, and the boiling point of water as 0. Academician P. S. Pallas in his expeditions of 1768-1774. in the Urals and Siberia I used the Deli thermometer. M.V. Lomonosov used in his research a thermometer he designed with a scale inverse to the Deli scale.
Thermometers were used primarily for meteorological and geophysical purposes. Lomonosov, who discovered the existence of vertical currents in the atmosphere, studying the dependence of the density of atmospheric layers on temperature, provides data from which it is possible to determine the coefficient of volumetric expansion of air, equal, according to these data, approximately ]/367. Lomonosov passionately defended the priority of the St. Petersburg academician Brown in the discovery of the freezing point of mercury, who on December 14, 1759 first froze mercury using cooling mixtures. This was the lowest temperature reached at that time.
The highest temperatures (without quantitative estimates) were obtained in 1772 by a commission of the Paris Academy of Sciences under the leadership of the famous chemist Lavoisier. High temperatures were obtained using a specially made lens. The lens was assembled from two concave-convex lentils, the space between which was filled with alcohol. About 130 liters of alcohol were poured into a lens with a diameter of 120 cm; its thickness reached 16 cm in the center. By focusing the sun's rays, it was possible to melt zinc, gold, and burn diamond. Both in the experiments of Brown-Lomonosov, where the “refrigerator” was winter air, and in the experiments of Lavoisier, the source of high temperatures was the natural “stove” - the Sun.
The development of thermometry was the first scientific and practical use thermal expansion of bodies. Naturally, the phenomenon of thermal expansion itself began to be studied not only qualitatively, but also quantitatively. The first accurate measurements of thermal expansion solids were performed by Lavoisier and Laplace in 1782. Their method was described for a long time in physics courses, starting with Biot’s course, 1819, and ending with O. D. Khvolson’s physics course, 1923.
A strip of the body being tested was placed first in melting ice and then in boiling water. Data were obtained for various types of glass, steel and iron, as well as for different types of gold, copper, brass, silver, tin, and lead. Scientists have found that depending on the method of preparing the metal, the results are different. A strip made of unhardened steel increases by 0.001079 of the original length when heated by 100°, and a strip of hardened steel increases by 0.001239. For wrought iron a value of 0.001220 was obtained, for round drawn iron it was 0.001235. These data give an idea of the accuracy of the method.
So, already in the first half of the 18th century, thermometers were created and quantitative thermal measurements began, brought to high degree accuracy in the thermophysical experiments of Laplace and Lavoisier. However, the basic quantitative concepts of thermophysics did not crystallize immediately. In the works of physicists of that time, there was considerable confusion in such concepts as “amount of heat”, “degree of heat”, “degree of heat”. The need to distinguish between the concepts of temperature and amount of heat was pointed out in 1755 by I. G. Lambert (1728-1777). However, his instructions were not appreciated by his contemporaries, and the development of correct concepts was slow.
The first approaches to calorimetry are contained in the works of St. Petersburg academicians G.V. Kraft and G.V. Richman (1711-1753). Craft's paper "Different Experiments with Heat and Cold," presented to the Academy Conference in 1744 and published in 1751, deals with the problem of determining the temperature of a mixture of two portions of liquid taken at different temperatures. This problem was often called “Richmann’s problem” in textbooks, although Richmann solved a more general and more difficult task than Kraft. Kraft gave an incorrect empirical formula to solve the problem.
We find a completely different approach to solving the problem in Richman. In the article “Reflections on the amount of heat that should be obtained by mixing liquids having certain degrees of heat,” published in 1750, Richmann poses the problem of determining the temperature of a mixture of several (and not two, as in Kraft) liquids and solves it based on principle heat balance. “Suppose,” says Richman, “that the mass of the liquid is equal to a; the heat distributed in this mass is equal to m; let the other mass, in which the same heat m be distributed as in mass a, be equal to a + b. Then the resulting heat
is equal to am/(a+b). Here Richmann understands temperature by “heat,” but the principle he formulated that “the same heat is inversely proportional to the masses over which it is distributed” is purely calorimetric. “Thus,” writes Richmann further, “the heat of mass a, equal to m, and the heat of mass b, equal to n, are uniformly distributed over mass a + b, and the heat in this mass, i.e., in a mixture of a and b, must be equal to the sum of heats m + n distributed in the mass a + b, or equal to (ma + nb) / (a + b) . This formula appeared in textbooks as the “Richmann formula”. “In order to obtain a more general formula,” continues Richman, “by which it would be possible to determine the degree of heat when mixing 3, 4, 5, etc. masses of the same liquid, having different degrees of heat, I called these masses a, b, c, d, e, etc., and the corresponding heats are m, n, o, p, q, etc. In exactly the same way I assumed that each of them is distributed over the totality of all masses.” As a result, “the heat after mixing all the warm masses is equal to:
(am + bп + с + dp + eq) etc./(a + b + c+d + e) etc.
that is, the sum of liquid masses, over which the heat of the individual masses is evenly distributed when mixed, is related to the sum of all products of each mass by its heat in the same way as unity is to the heat of the mixture.”
Richmann did not yet master the concept of the amount of heat, but he wrote and logically substantiated a completely correct calorimetric formula. He easily discovered that his formula agreed better with experience than Krafg’s formula. He correctly established that his “heats” were “not actual heat, but the excess heat of the mixture compared to zero degrees Fahrenheit.” He understood quite clearly that: 1. “The heat of the mixture is distributed not only throughout its mass itself, but also along the walls of the vessel and the thermometer itself.” 2. “The thermometer’s own heat and the heat of the vessel are distributed throughout the mixture, along the walls of the vessel in which the mixture is located, and along the thermometer.” 3. “Part of the heat of the mixture, during the period of time while the experiment is being carried out, passes into the surrounding air...”
Richman accurately formulated the sources of errors in calorimetric experiments, indicated the reasons for the discrepancy between Kraft's formula and experiment, i.e., he laid the foundations of calorimetry, although he himself had not yet approached the concept of the amount of heat. Richmann's work was continued by the Swedish academician Johann Wilcke (1732-1796) and the Scottish chemist Joseph Black (1728-1799). Both scientists, relying on Richmann’s formula, found it necessary to introduce new concepts into science. Wilke, while studying the heat of a mixture of water and snow in 1772, discovered that part of the heat disappears. Hence, he came to the concept of latent heat of snow melting and the need to introduce a new concept, which was later called “heat capacity.”
Black, who did not publish his results, came to the same conclusion. His research was published only in 1803, and then it became known that Black was the first to clearly distinguish between the concepts of quantity of heat and temperature, and the first to introduce the term “heat capacity.” Back in 1754-1755, Black discovered not only the constancy of the melting point of ice, but also that the thermometer remains at the same temperature, despite the influx of heat, until all the ice has melted. From here Black came to the concept of latent heat of fusion. Later he established the concept of latent heat of vaporization. Thus, by the 70s of the 18th century, the basic calorimetric concepts were established. Only almost a hundred years later (in 1852) was the unit of heat quantity introduced, which much later received the name “calorie.”( Clausius also speaks simply about the unit of heat and does not use the term “calorie”.)
In 1777, Lavoisier and Laplace, having built an ice calorimeter, determined the specific heat capacities of various bodies. Aristotle's primary quality, heat, began to be studied by precise experiment.
Appeared and scientific theories warmth. One, the most common concept (Black also adhered to it) is the theory of a special thermal fluid - caloric. The other, of which Lomonosov was a zealous supporter, considered heat as a type of movement of “insensitive particles.” The concept of caloric was very well suited to the description of calorimetric facts: Richmann's formula and later formulas taking into account latent heat could be perfectly explained. As a result, the theory of caloric dominated until the middle of the 19th century, when the discovery of the law of conservation of energy forced physicists to return to the concept successfully developed by Lomonosov another hundred years before the discovery of this law.
The idea that heat is a form of motion was very common in the 17th century. f. Bacon in the New Organon, applying his method to the study of the nature of heat, comes to the conclusion that “heat is a movement of propagation, hindered and occurring in small parts.” Descartes speaks more specifically and clearly about heat as the movement of small particles. Considering the nature of fire, he comes to the conclusion that “the body of the flame... is composed of the smallest particles, moving very quickly and violently separately from one another.” He further points out that “only this movement, depending on the various actions it produces, is called either heat or light.” Moving on to the rest of the bodies, he states “that small particles that do not stop their movement are present not only in fire, but also in all other bodies, although in the latter their action is not so strong, and due to their small size they themselves cannot to be noticed by any of our senses."
Atomism dominated the physical views of scientists and thinkers of the 17th century. Hooke, Huygens, Newton imagined all the bodies of the Universe as consisting of tiny particles, “insensitive”, as Lomonosov later briefly called them. The concept of heat as a form of movement of these particles seemed quite reasonable to scientists. But these ideas about warmth were qualitative character and arose on a very meager factual basis. In the 18th century knowledge about thermal phenomena became more accurate and definite; chemistry also made great strides, in which the theory of phlogiston, before the discovery of oxygen, helped to understand the processes of combustion and oxidation. All this contributed to the assimilation of a new point of view on heat as a special substance, and the first successes of calorimetry strengthened the position of supporters of caloric. It took great scientific courage to develop the kinetic theory of heat in this situation.
The kinetic theory of heat was naturally combined with the kinetic theory of matter, and above all air and vapor. Gases (the word “gas” was introduced by Van Helmont; 1577-1644) essentially had not yet been discovered, and even Lavoisier considered steam as a combination of water and fire. Lomonosov himself, observing the dissolution of iron in strong vodka ( nitric acid), believed
nitrogen bubbles released by air. Thus, air and steam were almost the only gases in Lomonosov’s time - “elastic liquids”, according to the terminology of that time.
D. Bernoulli in his “Hydrodynamics” imagined air as consisting of particles moving “extremely quickly in various directions”, and believed that these particles form an “elastic liquid”. Bernoulli substantiated the Boyle-Mariotte law with his model of “elastic fluid”. He established a connection between the speed of particle movement and the heating of air and thereby explained the increase in air elasticity when heated. This was the first attempt in the history of physics to interpret the behavior of gases by the movement of molecules, an undoubtedly brilliant attempt, and Bernoulli went down in the history of physics as one of the founders of the kinetic theory of gases.
Six years after the publication of Hydrodynamics, Lomonosov presented his work “Reflections on the Cause of Heat and Cold” to the Academic Assembly. It was published only six years later, in 1750, together with another, later work, “An Experience in the Theory of Air Elasticity.” Thus, Lomonosov's theory of elasticity of gases is inextricably linked with his theory of heat and is based on the latter.
D. Bernoulli also paid great attention to issues of heat, in particular the issue of the dependence of air density on temperature. Not limiting himself to referring to Amonton's experiments, he himself tried to experimentally determine the dependence of air elasticity on temperature. “I found,” writes Bernoulli, “that the elasticity of the air, which was very cold here in St. Petersburg on December 25, 1731 Art. Art., refers to the elasticity of the same air, which has the same heat as boiling water, as 523 to 1000.” This value from Bernoulli is clearly incorrect, since it assumes that the cold air temperature corresponds to - 78 ° C.
Lomonosov’s similar calculations, mentioned above, are much more accurate. But the final result of Bernoulli is very remarkable: “the elasticities are in the ratio composed of the square of the particle velocities and the first power of densities,” which is entirely consistent with the basic equation of the kinetic theory of gases in the modern presentation.
Bernoulli did not touch at all on the question of the nature of heat, which was central to Lomonosov’s theory. Lomonosov hypothesizes that heat is a form of motion of insensitive particles. He considers the possible nature of these movements: translational motion, rotational and oscillatory - and states that "heat consists in the internal rotational motion of bound matter."
Having accepted as an initial premise the hypothesis of the rotational motion of molecules as the cause of heat, Lomonosov deduces from this a number of consequences: 1) molecules (corpuscles) have a spherical shape; 2) “...with faster rotation of particles of bound matter, heat should increase, and with slower rotation, it should decrease; 3) particles of hot bodies rotate faster, colder ones - slower; 4) hot bodies must cool when in contact with cold ones, since it slows down the calorific movement of particles; on the contrary, cold bodies must heat up due to the acceleration of movement upon contact.” Thus, the transition of heat from a hot body to a cold body observed in nature is a confirmation of Lomonosov’s hypothesis.
The fact that Lomonosov singled out heat transfer as one of the main consequences is very significant, and some authors see this as a basis for classifying Lomonosov as the discoverer of the second law of thermodynamics. It is unlikely, however, that the above statement can be considered as the primary formulation of the second law, but the entire work as a whole is undoubtedly the first sketch of thermodynamics. Thus, Lomonosov explains in it the formation of heat during friction, which served as the experimental basis of the first law in Joule’s classical experiments. Lomonosov further, touching on the issue of the transfer of heat from a hot body to a cold one, refers to the following position: “Body A, acting on body B, cannot give the latter a greater speed of movement than what it itself has.” This position is a specific case of the “universal law of conservation”. Based on this position, he proves that a cold body B, immersed in a warm liquid A, “obviously cannot perceive a greater degree of heat than that of A.”
Lomonosov postpones the question of thermal expansion “until another time,” until he considers the elasticity of air. His thermodynamic work is thus directly adjacent to his later work on the elasticity of gases. However, speaking of his intention to postpone the consideration of thermal expansion “until another time,” Lomonosov here also points out that since there is no upper limit to the speed of particles (the theory of relativity does not yet exist!), then there is no upper limit to temperature. But “of necessity there must be a greatest and final degree of cold, which must consist in the complete cessation rotational movement particles." Lomonosov, therefore, asserts the existence of the “last degree of cold” - absolute zero.
In conclusion, Lomonosov criticizes the theory of caloric, which he considers a relapse of the ancient idea of elementary fire. Analyzing various phenomena, both physical and chemical, associated with the release and absorption of heat, Lomonosov concludes that “the heat of bodies cannot be attributed to the condensation of some thin, specially intended matter, but that heat consists in the internal rotational movement of the connected matter of the heated bodies." By “bound” matter, Lomonosov understands the matter of particles of bodies, distinguishing it from “flowing” matter, which can flow “like a river” through the pores of a body.
At the same time, Lomonosov included the world ether in his thermodynamic system, far ahead of not only his time, but also the 19th century. “Thereby,” continues Lomonosov, “we not only say that such movement and heat are also characteristic of that subtlest matter of the ether, which fills all spaces that do not contain sensitive bodies, but we also assert that the matter of the ether can impart the calorific motion received from the sun our earth and the rest of the bodies of the world and heat them, being the medium through which bodies distant from each other impart heat without the mediation of anything tangible.”
So, Lomonosov, long before Boltzmann, Golitsyn and Wien, included thermal radiation in thermodynamics. Lomonosov's thermodynamics is a remarkable achievement scientific thought XVIII century, far ahead of its time.
The question arises: why did Lomonosov refuse to consider the thermal translational motion of particles, but settled on rotational motion? This assumption greatly weakened his work, and D. Bernoulli's theory came much closer to the later studies of Clausius and Maxwell than Lomonosov's theory. Lomonosov had very deep thoughts on this matter. He had to explain such contradictory things as cohesion and elasticity, the coherence of body particles and the ability of bodies to expand. Lomonosov was an ardent opponent of long-range forces and could not resort to them when considering molecular structure tel. He also did not want to reduce the explanation of the elasticity of gases to elastic impacts of particles, that is, to explain elasticity by elasticity. He was looking for a mechanism that would explain both elasticity and thermal expansion in the most natural way. In his work “An Experience in the Theory of Air Elasticity,” he rejects the hypothesis of the elasticity of the particles themselves, which, according to Lomonosov, “are devoid of any physical composition and organized structure...” and are atoms. Therefore, the property of elasticity is not exhibited by individual particles that do not have any physical complexity and organized structure, but is produced by a collection of them. So, the elasticity of gas (air), according to Lomonosov, is “a property of a collective of atoms.” The atoms themselves, according to Lomonosov, “must be solid and have extension,” and he considers their shape “very close” to spherical. The phenomenon of heat arising from friction forces him to accept the hypothesis that “air atoms are rough.” The fact that the elasticity of air is proportional to density leads Lomonosov to conclude “that it comes from some direct interaction of its atoms.” But atoms, according to Lomonosov, cannot act at a distance, but act only upon contact. The compressibility of air proves the presence of empty spaces in it, which make it impossible for atoms to interact. From here Lomonosov comes to a dynamic picture, when the interaction of atoms is replaced in time by the formation of empty space between them, and the spatial separation of atoms is replaced by contact. “It is evident, then, that the individual atoms of the air, in disorderly alternation, collide with the nearest ones at insensitive intervals of time, and when some are in contact, others rebound from each other and collide with those closest to them, in order to rebound again; Thus, continually pushed away from each other by frequent mutual shocks, they tend to disperse in all directions.” Lomonosov sees elasticity in this scattering in all directions. “The force of elasticity consists in the tendency of air to spread in all directions.”
It is, however, necessary to explain why atoms bounce off each other when interacting. The reason for this, according to Lomonosov, is thermal motion: “The interaction of air atoms is caused only by heat.” And since heat consists in the rotational motion of particles, to explain their repulsion it is enough to consider what happens when two rotating spherical rough particles come into contact. Lomonosov shows that they will push away from each other, and illustrates this with the example of the rebound of tops (“head over heels”) that boys throw on ice, well known to him from childhood. When such spinning tops come into contact, they bounce away from each other over considerable distances. Thus, elastic collisions of atoms, according to Lomonosov, are caused by the interaction of their rotational moments. That's why he needed the hypothesis of thermal rotational motion of particles! Thus, Lomonosov completely substantiated the model of an elastic gas consisting of chaotically moving and colliding particles.
This model allowed Lomonosov not only to explain the Boyle-Mariotte law, but also to predict deviations from it under large compressions. An explanation of the law and deviations from it was given by Lomonosov in his work “Addition to Reflections on the Elasticity of Air,” published in the same volume of “New Commentaries” of the St. Petersburg Academy of Sciences in which the two previous works were published. In Lomonosov's works there are also incorrect statements, which can be fully explained by the level of knowledge of that time. But they do not determine the significance of a scientist’s work. One cannot help but admire the courage and depth of Lomonosov’s scientific thought, who, in the infancy of the science of heat, created a powerful theoretical concept that was far ahead of its era. Contemporaries did not follow the path of Lomonosov; in the theory of heat, as was said, caloric reigned; the physical thinking of the 18th century required various substances: thermal, light, electrical, magnetic. Usually this is seen as the metaphysical nature of the thinking of natural scientists of the 18th century, and some of its reactionary nature. But why did it become like this? It seems that the reason for this lies in the progress of exact natural science. In the 18th century learned to measure heat, light, electricity, magnetism. Measures have been found for all these agents, just as they were found long ago for ordinary masses and volumes. This fact brought weightless agents closer to ordinary masses and liquids and forced them to be considered as an analogue of ordinary liquids. The concept of “weightless” was a necessary stage in the development of physics; it made it possible to penetrate deeper into the world of thermal, electrical and magnetic phenomena. She contributed to the development of precise experimentation, the accumulation of numerous facts and their primary interpretation.
Temperature scales. There are several graduated temperature scales, and the freezing and boiling temperatures of water are usually taken as reference points. Now the most common scale in the world is the Celsius scale. In 1742, Swedish astronomer Anders Celsius proposed a 100-degree thermometer scale in which 0 degrees is the boiling point of water at normal atmospheric pressure and 100 degrees is the melting temperature of ice. The scale division is 1/100 of this difference. When thermometers began to be used, it turned out to be more convenient to swap 0 and 100 degrees. Perhaps Carl Linnaeus participated in this (he taught medicine and natural science at the same University of Uppsala where Celsius taught astronomy), who back in 1838 proposed taking the melting temperature of ice as 0 temperature, but apparently did not think of a second reference point. By now, the Celsius scale has changed somewhat: 0°C is still taken to be the melting temperature of ice at normal pressure, which is not very dependent on pressure. But the boiling point of water at atmospheric pressure is now 99,975°C, which does not affect the measurement accuracy of almost all thermometers except special precision ones. The Fahrenheit temperature scales of Kelvin Reaumur and others are also known. The Fahrenheit temperature scale (in the second version adopted since 1714) has three fixed points: 0° corresponded to the temperature of a mixture of ice water and ammonia 96° - the body temperature of a healthy person (under the armpit or in the mouth ). The reference temperature for comparing various thermometers was taken to be 32° for the melting point of the ice. The Fahrenheit scale is widely used in English-speaking countries but is almost never used in scientific literature. To convert Celsius temperature (°C) to Fahrenheit temperature (°F) there is a formula °F = (9/5)°C + 32 and for the reverse conversion there is a formula °C = (5/9)(°F-32) ). Both scales - both Fahrenheit and Celsius - are very inconvenient when conducting experiments in conditions where the temperature drops below the freezing point of water and is expressed negative number. For such cases, absolute temperature scales were introduced, which are based on extrapolation to the so-called absolute zero - the point at which molecular motion should stop. One of them is called the Rankine scale and the other is the absolute thermodynamic scale; temperatures are measured in degrees Rankine (°Ra) and kelvins (K). Both scales begin at absolute zero temperature and the freezing point of water corresponds to 491 7° R and 273 16 K. The number of degrees and kelvins between the freezing and boiling points of water on the Celsius scale and the absolute thermodynamic scale is the same and equal to 100; for the Fahrenheit and Rankine scales it is also the same but equal to 180. Celsius degrees are converted to kelvins using the formula K = °C + 273 16 and Fahrenheit degrees are converted to Rankine degrees using the formula °R = °F + 459 7. has been common in Europe for a long time Reaumur scale introduced in 1730 by Rene Antoine de Reaumur. It is not built arbitrarily like the Fahrenheit scale, but in accordance with the thermal expansion of alcohol (in a ratio of 1000:1080). 1 degree Reaumur is equal to 1/80 of the temperature interval between the points of melting ice (0°R) and boiling water (80°R) i.e. 1°R = 1.25°C 1°C = 0.8°R. but has now fallen into disuse.
ABSOLUTE TEMPERATURE SCALE.
1.
Temperature
is a measure of the average kinetic energy of molecules, characterizing
degree of heating of bodies.
2. Temperature measuring device - thermometer .
3.
Operating principle
thermometer:
When measuring temperature, the dependence of changes in any macroscopic parameter (volume, pressure, electrical resistance etc.) substances depending on temperature.
In liquid thermometers, this is a change in the volume of liquid.
When two media come into contact, energy is transferred from the more heated environment to the less heated one.
During the measurement process, the body temperature and the thermometer reach a state of thermal equilibrium.
Thermometers.
In practice, liquid thermometers are often used: mercury (in the range from -35 C to +750 C) and alcohol (from -80 C to +70 C).
They use the property of a liquid to change its volume when the temperature changes.
However, each liquid has its own characteristics of volume change (expansion) at different temperatures.
As a result of comparing, for example, the readings of mercury and alcohol thermometers, an exact match will be only at two points (at temperatures of 0 C and 100 C).
These disadvantages are absentgas thermometers
.
The first gas thermometer was created by the French. physicist J. Charles.
When two bodies of different temperatures come into contact, internal energy is transferred from the more heated body to the less heated one, and the temperatures of both bodies are equalized.
A state of thermal equilibrium occurs in which all macroparameters (volume, pressure, temperature) of both bodies subsequently remain unchanged under constant external conditions.
4.
Thermal equilibrium
is a state in which all macroscopic parameters remain unchanged for an indefinitely long time.
5. The state of thermal equilibrium of a system of bodies is characterized by temperature: all bodies of the system that are in thermal equilibrium with each other have the same temperature.
where k is Boltzmann’s constant
![]()
This dependence makes it possible to introduce a new temperature scale– an absolute temperature scale, independent of the substance used to measure temperature.
6.Absolute temperature scale
- English introduced physicist W. Kelvin
- no negative temperatures
SI unit of absolute temperature: [T] = 1K (Kelvin)
The zero temperature of the absolute scale is absolute zero (0K = -273 C), the most low temperature in nature. ABSOLUTE ZERO is the extremely low temperature at which the thermal movement of molecules stops.

Relationship between the absolute scale and the Celsius scale
In formulas, absolute temperature is denoted by the letter “T”, and temperature on the Celsius scale by the letter “t”.
![]()
History of invention thermometer
The inventor of the thermometer is considered to be : in his own writings there is no description of this device, but his students, Nelly and , testified that already in he made something like a thermobaroscope ( ). Galileo studied at this time the work , who has already described a similar device, but not for measuring degrees of heat, but for raising water by heating. The thermoscope was a small glass ball with a glass tube soldered to it. The ball was slightly heated and the end of the tube was lowered into a vessel with water. After some time, the air in the ball cooled, its pressure decreased and the water, under the influence of atmospheric pressure, rose up in the tube to a certain height. Subsequently, with warming, the air pressure in the ball increased and the water level in the tube decreased as it cooled, but the water in it rose. Using a thermoscope, it was possible to judge only the change in the degree of heating of the body: it did not show numerical temperature values, since it did not have a scale. In addition, the water level in the tube depended not only on temperature, but also on atmospheric pressure. In 1657, Galileo's thermoscope was improved by Florentine scientists. They equipped the device with a bead scale and pumped out the air from the reservoir (ball) and tube. This made it possible not only to qualitatively, but also quantitatively compare body temperatures. Subsequently, the thermoscope was changed: it was turned upside down, and instead of water, alcohol was poured into the tube and the vessel was removed. The action of this device was based on the expansion of bodies; the temperatures of the hottest summer and coldest temperatures were taken as “constant” points. winter day. The invention of the thermometer is also attributed to Lord , , Sanctorius, Scarpi, Cornelius Drebbel ( ), Porte and Salomon de Caus, who wrote later and partly had personal relations with Galileo. All these thermometers were air thermometers and consisted of a vessel with a tube containing air separated from the atmosphere by a column of water; they changed their readings both from changes in temperature and from changes in atmospheric pressure.
Liquid thermometers were described for the first time in d. "Saggi di naturale esperienze fatte nell'Accademia del Cimento", where they are spoken of as objects that have long been made by skilled artisans, who are called "Confia", who heat the glass on the blown fire of a lamp and make amazing and very delicate products from it. At first these thermometers were filled with water, and they burst when it froze; they began to use wine alcohol for this in 1654 according to the idea of the Grand Duke of Tuscany . The Florentine thermometers are not only depicted in the Saggi, but have been preserved in several copies to this day in the Galilean Museum, in Florence; their preparation is described in detail.
First, the master had to make divisions on the tube, taking into account its relative sizes and the dimensions of the ball: the divisions were applied with molten enamel onto the tube heated in a lamp, every tenth was indicated by a white dot, and the others by black. They usually made 50 divisions in such a way that when the snow melts, the alcohol does not fall below 10, and in the sun does not rise above 40. Good craftsmen made such thermometers so successfully that they all showed the same temperature value under the same conditions, but this was not the case could be achieved if the tube was divided into 100 or 300 parts in order to obtain greater accuracy. The thermometers were filled by heating the ball and lowering the end of the tube into alcohol; the filling was completed using a glass funnel with a thin end that fit freely into a fairly wide tube. After adjusting the amount of liquid, the opening of the tube was sealed with sealing wax, called "sealant". From this it is clear that these thermometers were large and could be used to determine air temperature, but they were still inconvenient for other, more diverse experiments, and the degrees of different thermometers were not comparable with each other.
IN G. ( ) V improved the air thermometer, measuring not the expansion, but the increase in elasticity of air brought to the same volume at different temperatures by adding mercury to an open elbow; barometric pressure and its changes were taken into account. The zero of such a scale was supposed to be “that significant degree of cold” at which the air loses all its elasticity (that is, modern ), and the second constant point is the boiling point of water. The effect of atmospheric pressure on the boiling point was not yet known to Amonton, and the air in his thermometer was not freed from water gases; therefore, from his data, absolute zero is obtained at −239.5° Celsius. Another air thermometer of Amonton, made very imperfectly, was independent of changes in atmospheric pressure: it was a siphon barometer, the open elbow of which was extended upward, filled with a strong solution of potash at the bottom, oil at the top and ended in a sealed tank with air.
Modern shape added to the thermometer and described his method of preparation in 1723. Initially, he also filled his pipes with alcohol and only finally switched to mercury. He set the zero of his scale at the temperature of a mixture of snow with ammonia or table salt, at the temperature of “the beginning of freezing of water” he showed 32°, and the body temperature of a healthy person in the mouth or under the armpit was equivalent to 96°. Subsequently, he found that water boils at 212° and this temperature was always the same under the same condition . Surviving examples of Fahrenheit thermometers are distinguished by their meticulous execution.
The Swedish astronomer, geologist and meteorologist finally established both constant points, melting ice and boiling water. in 1742. But initially he set 0° at the boiling point, and 100° at the freezing point. In his work Celsius " "talked about his experiments showing that the melting temperature of ice (100°) does not depend on pressure. He also determined with amazing precision how the boiling point of water varied depending on . He suggested that mark 0 ( water) can be calibrated by knowing at what level relative to the sea the thermometer is located.
Later, after the death of Celsius, his contemporaries and compatriots botanist and astronomer Morten Stremer used this scale inverted (they began to take the melting temperature of ice as 0°, and the boiling point of water as 100°). In this form It turned out to be very convenient, became widespread and is used to this day.
According to some sources, Celsius himself turned his scale upside down on the advice of Stremer. According to other sources, the scale was turned over by Carl Linnaeus in 1745. And according to the third, the scale was turned upside down by Celsius’ successor M. Stremer, and in the 18th century such a thermometer was widely distributed under the name “Swedish thermometer”, and in Sweden itself - under the name Stremer, but the famous Swedish chemist Johann Jacob in his work “Manuals of Chemistry” mistakenly called M. Stremer's scale the Celsius scale, and since then the centigrade scale began to bear the name of Anders Celsius.
Works in 1736, although they led to the establishment of an 80° scale, they were rather a step back against what Fahrenheit had already done: Reaumur’s thermometer was huge, inconvenient to use, and its method of dividing into degrees was inaccurate and inconvenient.
After Fahrenheit and Reaumur, the business of making thermometers fell into the hands of artisans, as thermometers became an item of trade.
In 1848, the English physicist (Lord Kelvin) proved the possibility of creating an absolute temperature scale, the zero of which does not depend on the properties of water or the substance filling the thermometer. The starting point in " " served the meaning : −273.15° C. At this temperature, the thermal movement of molecules stops. Consequently, further cooling of the bodies becomes impossible.
Liquid thermometers
Liquid thermometers are based on the principle of changing the volume of liquid that is poured into the thermometer (usually or ), when the ambient temperature changes.
Due to the ban on the use of mercury in many areas of activity, alternative fillings for household thermometers are being sought. For example, such a replacement could be an alloy .
For information on removing spilled mercury from a broken thermometer, see the article
Mechanical thermometers
This type of thermometer operates on the same principle as electronic thermometers, but the sensor is usually spiral or .
Electric thermometers
The operating principle of electric thermometers is based on changing contact potential difference depending on temperature). The most accurate and stable over time are based on platinum wire or platinum coating on ceramics.
Optical thermometers
Optical thermometers allow you to record temperature by changing
Infrared thermometers
An infrared thermometer allows you to measure temperature without direct contact with a person. In some countries, there has long been a tendency to abandon mercury thermometers in favor of infrared ones, not only in medical institutions, but also at the household level.
Technical thermometers
Technical thermometers are used in enterprises in agriculture, petrochemical, chemical, mining and metallurgical industries, mechanical engineering, housing and communal services, transport, construction, medicine, in a word, in all spheres of life.
There are the following types of technical thermometers:
technical liquid thermometers TTZh-M;
bimetallic thermometers TB, TBT, TBI;
agricultural thermometers TS-7-M1;
maximum thermometers SP-83 M;
low-degree thermometers for special chambers SP-100;
special vibration-resistant thermometers SP-V;
mercury thermometers, electric contact TPK;
laboratory thermometers TLS;
thermometers for petroleum products TN;
thermometers for testing petroleum products TIN1, TIN2, TIN3, TIN4.
Now all we need is snow, a cup, a thermometer and a little patience. Let's bring a cup of snow from the frost, put it in a warm, but not hot place, immerse a thermometer in the snow and watch the temperature. At first, the mercury column will creep upward relatively quickly. The snow remains dry. Having reached zero, the mercury column will stop. From this moment the snow begins to melt. Water appears at the bottom of the cup, but the thermometer still shows zero. By continuously mixing the snow, it is not difficult to make sure that until it all melts, the mercury will not budge.
What causes the temperature to stop and just at the time when the snow turns into water? The heat supplied to the cup is entirely spent on the destruction of snowflake crystals. And as soon as the last crystal collapses, the water temperature will begin to rise.
The same phenomenon can be observed when melting any other crystalline substances. They all require some amount of heat to change from solid to liquid. This amount, quite specific for each substance, is called the heat of fusion.
The heat of fusion for different substances different. And it is here that, when we begin to compare the specific heats of fusion for various substances, water again stands out among them. Like specific heat, the specific heat of fusion of ice is much greater than the heat of fusion of any other substance.
To melt one gram of benzene, you need 30 calories, the heat of fusion of tin is 13 calories, lead - about 6 calories, zinc - 28, copper - 42 calories. And to turn ice into water at zero degrees requires 80 calories! This amount of heat is enough to raise the temperature of one gram of liquid water from 20 degrees to boiling. Only one metal, aluminum, has a specific heat of fusion that exceeds the heat of fusion of ice.
So, water at zero degrees differs from ice at the same temperature in that each gram of water contains 80 calories more heat than a gram of ice.
Now that we know how high the heat of fusion of ice is, we see that we have no reason to sometimes complain that ice melts “too quickly.” If ice had the same heat of fusion as most other bodies, it would melt several times faster.
In the life of our planet, the melting of snow and ice is of absolutely exceptional importance. It must be remembered that the ice sheet alone occupies more than three percent of the entire earth's surface or 11 percent of the entire landmass. In the area south pole lies the huge continent of Antarctica, larger in size than Europe and Australia combined, covered with a continuous layer of ice. Over millions of square kilometers of land reigns permafrost. Glaciers and permafrost alone make up a fifth of the landmass. To this we must add the surface included in winter time snow. And then we can say that from one quarter to one third of the land is always covered with ice and snow. Several months of the year this area exceeds half of the entire landmass.
It is clear that huge masses of frozen water cannot but affect the Earth's climate. What a colossal amount of solar heat is spent just to melt one snow cover in the spring! After all, on average it reaches about 60 centimeters in thickness, and for each gram you need to spend 80 calories. But the sun is such a powerful source of energy that in our latitudes it sometimes copes with this work in several days. And it’s hard to imagine what kind of flood would await us if ice had, for example, the same heat of fusion as lead. All the snow could melt in one day or even in a few hours, and then the rivers, swollen to extraordinary sizes, would wash away both the most fertile layer of soil and plants from the surface of the earth, bringing untold disasters to all life on Earth.
Ice, when melting, absorbs a huge amount of heat. The same amount of heat is released by water when it freezes. If water had a small heat of fusion, then our rivers, lakes and seas would probably freeze after the first frost.
So, in addition to the high heat capacity of water, another remarkable feature has been added - a high heat of fusion.