4. Nature and types of chemical bonds. Covalent bond
Application. Spatial structure molecules
Each molecule (for example, CO 2, H 2 O, NH 3) or molecular ion (for example, CO 3 2 −, H 3 O +, NH 4 +) has a certain qualitative and quantitative composition, as well as structure (geometry). Molecule geometry is formed due to a fixed relative position atoms and bond angle values.
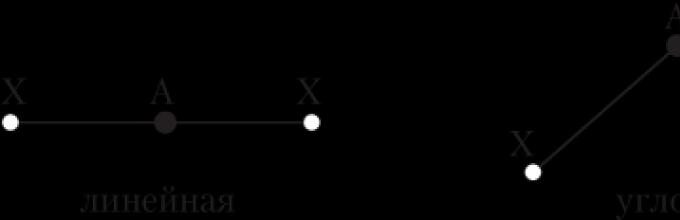
The bond angle is the angle between imaginary straight lines passing through the nuclei of chemically bonded atoms. We can also say that it is the angle between two bond lines that have a common atom.
A bond line is a line connecting the nuclei of two chemically bonded atoms.
Only in the case of diatomic molecules (H 2, Cl 2, etc.) the question of their geometry does not arise - they are always linear, i.e. the nuclei of atoms are located on the same straight line. The structure of more complex molecules may resemble different geometric shapes, For example:
- triatomic molecules and ions of the AX 2 type (H 2 O, CO 2, BeCl 2)

- tetraatomic molecules and ions of type AX 3 (NH 3, BF 3, PCl 3, H 3 O +, SO 3) or A 4 (P 4, As 4)

- pentaatomic molecules and ions of the AX 4 type (CH 4, XeF 4, GeCl 4)

There are particles and more complex structure(octahedron, trigonal bipyramid, flat regular hexagon). In addition, molecules and ions can have the shape of a distorted tetrahedron, an irregular triangle; in molecules of angular structure, the values of α can be different (90°, 109°, 120°).
The structure of molecules is reliably established experimentally using various physical methods. To explain the reasons for the formation of a particular structure and predict the geometry of molecules, various theoretical models have been developed. The easiest to understand are the model of repulsion of valence electron pairs (OVEP model) and the model of hybridization of valence atomic orbitals (GVAO model).
The basis of all (including the two mentioned) theoretical models that explain the structure of molecules is the following proposition: the stable state of a molecule (ion) corresponds to a spatial arrangement of atomic nuclei in which the mutual repulsion of electrons in the valence layer is minimal.
This takes into account the repulsion of electrons both participating in the formation of a chemical bond (bond electrons) and those not participating (lone pairs of electrons). It is taken into account that the orbital of a bonding electron pair is compactly concentrated between two atoms and therefore occupies less space than the orbital of a lone pair of electrons. For this reason, the repulsive effect of a nonbonding (lone) pair of electrons and its effect on bond angles is more pronounced than that of a bonding one.
OVEP model. This theory is based on the following basic principles (stated in a simplified manner):
- the geometry of the molecule is determined only by σ-bonds (but not π-);
- The angles between bonds depend on the number of lone pairs of electrons in the central atom.
These provisions should be considered together, since both the electrons of a chemical bond and lone pairs of electrons repel each other, which ultimately leads to the formation of a molecular structure in which this repulsion is minimal.
Let us consider the geometry of some molecules and ions from the standpoint of the OVEP method; electrons of a σ bond will be denoted by two dots (:), lone pairs of electrons by a conventional symbol ( or ) or a dash.
Let's start with the five-atom molecule of methane CH 4. In this case, the central atom (this carbon) has completely exhausted its valence capabilities and does not contain lone pairs of valence electrons, i.e. all four valence electrons form four σ bonds. How should the electrons of the σ bond be positioned relative to each other so that the repulsion between them is minimal? Obviously, at an angle of 109°, i.e. along lines directed to the vertices of an imaginary tetrahedron, in the center of which is a carbon atom. In this case, the electrons taking part in the formation of the bond are as far apart from each other as possible (for a square configuration, the distance between these bond electrons is greater and the interelectron repulsion is less). For this reason, the methane molecule, as well as the molecules CCl 4, CBr 4, CF 4, have the form regular tetrahedron(said to have a tetrahedral structure):

The ammonium cation NH + 4 and the anion BF 4 − have the same structure, since the nitrogen and boron atoms form four σ bonds each, and they do not have lone pairs of electrons.
Let's consider the structure of the tetraatomic ammonia molecule NH 3. The ammonia molecule has three pairs of bonding electrons and one lone pair of electrons on the nitrogen atom, i.e. also four pairs of electrons. However, will the bond angle remain at 109°? No, since a lone pair of electrons, occupying a larger volume in space, has a strong repulsive effect on the electrons of the σ bond, which leads to a slight decrease in the bond angle, in this case this angle is approximately 107°. The ammonia molecule has the shape of a trigonal pyramid (pyramidal structure):

The tetraatomic hydronium ion H 3 O + also has a pyramidal structure: the oxygen atom forms three σ bonds and contains one lone pair of electrons.
In the four-atom BF 3 molecule, the number of σ bonds is also three, but the boron atom has no lone pairs of electrons. Obviously, the interelectron repulsion will be minimal if the BF 3 molecule has the shape of a regular flat triangle with a bond angle of 120°:

The molecules BCl 3, BH 3, AlH 3, AlF 3, AlCl 3, SO 3 have the same structure and for the same reasons.
What structure will a water molecule have?
A triatomic water molecule has four pairs of electrons, but only two of them are σ-bond electrons, the remaining two are lone pairs of electrons of the oxygen atom. The repulsive effect of two lone pairs of electrons in an H 2 O molecule is stronger than in an ammonia molecule with one lone pair, therefore the H–O–H bond angle is smaller than the H–N–H angle in an ammonia molecule: in a water molecule the bond angle is approximately 105° :

The CO 2 molecule (O=C=O) also has two pairs of bonding electrons (we consider only σ bonds), however, unlike a water molecule, the carbon atom does not have lone pairs of electrons. Obviously, the repulsion between pairs of electrons in this case will be minimal if they are located at an angle of 180°, i.e. at linear form CO 2 molecules:
The molecules BeH 2, BeF 2, BeCl 2 have a similar structure and for the same reasons. In a triatomic SO 2 molecule, the central atom (sulfur atom) also forms two σ bonds, but has a lone pair of electrons, therefore the sulfur(IV) oxide molecule has an angular structure, but the bond angle in it is greater than in a water molecule (the oxygen atom two lone pairs of electrons, and the sulfur atom has only one):

Some triatomic molecules of the ABC composition (for example, H–C≡N, Br–C≡N, S=C=Te, S=C=O) also have a linear structure, in which the central atom does not have lone pairs of electrons. But the HClO molecule has an angular structure (α ≈ 103°), since the central atom, the oxygen atom, contains two lone pairs of electrons.
Using the OVEP model, you can also predict the structure of molecules organic matter. For example, in the acetylene molecule C 2 H 2, each carbon atom forms two σ bonds, and the carbon atoms do not have lone pairs of electrons; therefore, the molecule has a linear structure H–C≡C–H.
In the ethene molecule C 2 H 4, each carbon atom forms three σ bonds, which, in the absence of lone pairs of electrons on the carbon atoms, leads to a triangular arrangement of atoms around each carbon atom:
In table 4.2 summarizes some data on the structure of molecules and ions.
Table 4.2
Relationship between the structure of molecules (ions) and the number σ -bonds and lone pairs of electrons of the central atom
| Type of molecule (ion) | Number of σ bonds formed by the central atom | Number of lone pairs of electrons | Structure, bond angle | Examples of particles (central atom highlighted) |
|---|---|---|---|---|
| AB 2 | 2 | 0 | Linear, α = 180° | CO 2 , Be H 2 , HC N , Be Cl 2 , C 2 H 2 , N 2 O , C S 2 |
| 1 | Angular, 90°< α < 120° | Sn Cl 2, S O 2, N O 2 − | ||
| 2 | Angular, α< 109° | H 2 O , O F 2 , H 2 S , H 2 Se , S F 2 , Xe O 2 , − | ||
| AB 3 | 3 | 0 | Triangular, α ≈ 120° | B F 3, B H 3, B Cl 3, Al F 3, S O 3, C O 3 2 −, N O 3 − |
| 1 | Trigonal pyramid, α< 109° | N H 3 , H 3 O + , N F 3 , S O 3 2 − , P F 3 , P Cl 3 , As H 3 | ||
| AB 4 | 4 | 0 | Tetrahedron, α = 109° | N H 4 + , CH 4 , Si H 4 , B F 4 , B H 4 − , S O 4 2 − , A l H 4 − |
GVAO model. The main position of this model is that the formation of covalent bonds involves not “pure” valence s -, p - and d - orbitals, but the so-called hybrid orbitals. Next, hybridization only involving 2p- and 2s-AOs is considered.
Hybridization is the phenomenon of mixing of valence orbitals, as a result of which they align in shape and energy.
The concept of hybridization is always used when electrons of different energy sublevels that do not differ very much in energy participate in the formation of chemical bonds: 2s and 2p, 4s, 4p and 3d, etc.
The hybrid orbital is not similar in shape to the original 2p- and 2s-AO. It has the shape of an irregular three-dimensional figure eight:
As we can see, hybrid AOs are more elongated, so they can overlap better and form stronger covalent bonds. When hybrid orbitals overlap, only σ bonds are formed; Due to their specific shape, hybrid AOs do not participate in the formation of π-bonds (only non-hybrid AOs form π-bonds). The number of hybrid orbitals is always equal to the number of initial AOs participating in hybridization. Hybrid orbitals must be oriented in space in such a way as to ensure their maximum distance from each other. In this case, the repulsion of the electrons located on them (bonding and non-bonding) will be minimal; the energy of the entire molecule will also be minimal.
The GVAO model assumes that hybridization involves orbitals with similar energy values (i.e., valence orbitals) and a sufficiently high electron density. The electron density of an orbital decreases as its size increases, so the role in hybridization is especially significant for molecules of elements of small periods.
It should be remembered that GVAO is not real physical phenomenon, and a convenient concept ( mathematical model), which allows us to describe the structure of some molecules. The formation of hybrid AOs is not detected by any physical methods. Nevertheless, the theory of hybridization has some physical justification.
Let's consider the structure of the methane molecule. It is known that the CH4 molecule has the shape of a regular tetrahedron with a carbon atom in the center; all four C–H bonds are formed by an exchange mechanism and have the same energy and length, i.e. are equivalent. Explain the presence of four carbon atoms unpaired electrons quite simply, assuming its transition to an excited state:

However, this process does not in any way explain the equivalence of all four C–H bonds, since according to the above scheme, three of them are formed with the participation of the 2p-AO of the carbon atom, one with the participation of the 2s-AO, and the shape and energy of the 2p and 2s-AO are different.
To explain this and other similar facts, L. Pauling developed the concept of GVAO. It is assumed that mixing of orbitals occurs at the moment of formation of chemical bonds. This process requires energy expenditure for the pairing of electrons, which, however, is compensated by the release of energy when hybrid AOs form stronger (compared to non-hybrid) bonds.
Based on the nature and number of AOs involved in hybridization, several types are distinguished.
In the case of sp 3 hybridization, one s and three p orbitals are mixed (hence the name of the type of hybridization). For a carbon atom, the process can be represented as follows:
1 s 2 2 s 2 2 p x 1 2 p y 1 → electron transition 1 s 2 2 s 1 2 p x 1 2 p y 1 2 p z 1 → hybridization 1 s 2 2 (s p 3) 4
or using electronic configurations:

Four sp 3 -hybrid AOs occupy an intermediate position in energy between 2p and 2s AOs.
The sp 3 hybridization scheme can be represented using images of the AO shape of the carbon atom:

Thus, as a result of sp 3 hybridization, four hybrid orbitals are formed, each of which contains an unpaired electron. These orbitals are located at an angle of 109°28′ in space, which ensures minimal repulsion of the electrons located on them. If you connect the vertices of hybrid orbitals, you get a three-dimensional figure - a tetrahedron. For this reason, molecules of the composition AX 4 (CH 4, SiH 4, CCl 4, etc.), in which this type of hybridization is realized, have the shape of a tetrahedron.
The concept of sp 3 hybridization of AO also explains well the structure of H 2 O and NH 3 molecules. It is assumed that 2s - and 2p -AOs of nitrogen and oxygen atoms participate in hybridization. In these atoms, the number of valence electrons (5 and 6, respectively) exceeds the number of sp 3 -hybrid AOs (4), therefore, some hybrid AOs contain unpaired electrons, and some contain lone pairs of electrons:

We see that in the nitrogen atom the lone pair of electrons is located on one hybrid AO, and in the oxygen atom - on two. Only AOs with unpaired electrons participate in the formation of bonds with hydrogen atoms, and lone pairs of electrons will have a repulsive effect (Fig. 4.5) on each other (in the case of oxygen) and on the bonding electrons (for oxygen and nitrogen).

Rice. 4.5. Scheme of the repulsive action of bonding and nonbonding orbitals in the molecule of ammonia (a) and water (b)
The repulsion is stronger in the case of the water molecule. Since the oxygen atom has two lone pairs of electrons, the deviation from the ideal value of the bond angle for this type of hybridization (109°28′) in a water molecule is greater than in an ammonia molecule (in H 2 O and NH 3 molecules the bond angle is 104, respectively ,5° and 107°).
The sp 3 hybridization model is used to explain the structure of diamond, silicon, NH 4 + and H 3 O + ions, alkanes, cycloalkanes, etc. In the case of carbon, this type of hybridization is always used when an atom of this element forms only σ bonds.
In the case of sp 2 hybridization, one s and two p orbitals are mixed. Let us consider this type of hybridization using the example of a boron atom. The process is represented using energy diagrams


Thus, as a result of sp 2 hybridization of the valence orbitals of the boron atom, three hybrid AOs are formed, directed at an angle of 120°, and one of the 2p orbitals does not take part in hybridization. Hybrid orbitals contain one unpaired electron, are located in the same plane, and if you connect their vertices, you get regular triangle. For this reason, molecules of the composition AX 3 with sp 2 hybridization of the orbitals of atom A have a triangular structure, as shown for the BF 3 molecule:

The non-hybrid 2p-AO of the boron atom is free (unoccupied) and oriented perpendicular to the plane of B–F bonds, therefore the BF 3 molecule is an electron acceptor when forming a covalent bond according to the donor-acceptor mechanism when interacting with an ammonia molecule.
The concept of sp 2 hybridization is used to explain the nature of the carbon-carbon double bond in alkenes, the structure of benzene and graphite, i.e. in cases where the carbon atom forms three σ and one π bond.
The spatial arrangement of the orbitals of the carbon atom for sp 2 hybridization looks like this: the non-hybrid 2p AO is oriented perpendicular to the plane in which the hybrid orbitals are located (both hybrid and non-hybrid AO contain an unpaired electron).

Let's consider the formation of chemical bonds in the ethylene molecule H 2 C=CH 2. In it, the hybrid AOs overlap with each other and with the 1s-AOs of the hydrogen atom, forming five σ bonds: one C–C and four C–H. Non-hybrid 2p-AOs overlap laterally and form a π bond between carbon atoms (Fig. 4.6).

Rice. 4.6. Scheme of formation of σ-bonds (a) and π-bond (b) in an ethylene molecule
In the case of sp hybridization, one s and one p orbital are mixed. Let us consider this type of hybridization using the example of the beryllium atom. Let's imagine the hybridization process using an energy diagram:

and with an image of the shape of the orbitals
Thus, as a result of sp-hybridization, two hybrid AOs are formed, each containing one unpaired electron. Two 2p-AOs do not take part in hybridization and, in the case of beryllium, remain vacant. Hybrid orbitals are oriented at an angle of 180°, therefore molecules of the AX 2 type with sp-hybridization of the orbitals of atom A have a linear structure (Fig. 4.7).

Rice. 4.7. Spatial structure of the BeCl 2 molecule
Using the model of sp-hybridization of the orbitals of the carbon atom, the nature of the triple bond in alkyne molecules is explained. In this case, two hybrid and two non-hybrid 2p-AOs (shown by horizontal arrows →, ←) each contain an unpaired electron:

In the acetylene molecule HC≡CH, due to hybrid AOs, σ-bonds C–H and C–C are formed:

Hybrid 2p-AOs overlap in two perpendicular planes and form two π bonds between carbon atoms (Fig. 4.8).

Rice. 4.8. Schematic representation of π-bonds (a) and planes of π-bonds (b) in the acetylene molecule (the wavy line shows the lateral overlap of the 2p-AO of the carbon atom)
The concept of sp-hybridization of the orbitals of the carbon atom makes it possible to explain the formation of chemical bonds in carbyne, CO and CO 2 molecules, propadiene (CH 2 =C=CH 2), i.e. in all cases where a carbon atom forms two σ and two π bonds.
The main characteristics of the considered types of hybridization and the geometric configurations of molecules corresponding to some types of hybridization of the orbitals of the central atom A (taking into account the influence of non-bonding electron pairs) are presented in Table. 4.3 and 4.4.
Table 4.3
Main Features different types hybridization
Comparing the data in Table. 4.2 and 4.4, we can conclude that both models - OVEP and GVAO - lead to the same results regarding the structure of the molecules.
Table 4.4
Types of spatial configuration of molecules corresponding to certain types of hybridization
Chemical bond
All interactions leading to the combination of chemical particles (atoms, molecules, ions, etc.) into substances are divided into chemical bonds and intermolecular bonds (intermolecular interactions).
Chemical bonds- bonds directly between atoms. There are ionic, covalent and metallic bonds.
Intermolecular bonds- connections between molecules. These are hydrogen bonds, ion-dipole bonds (due to the formation of this bond, for example, the formation of a hydration shell of ions occurs), dipole-dipole (due to the formation of this bond, molecules of polar substances are combined, for example, in liquid acetone), etc.
Ionic bond- a chemical bond formed due to the electrostatic attraction of oppositely charged ions. In binary compounds (compounds of two elements), it is formed when the sizes of the bonded atoms differ greatly from each other: some atoms are large, others are small - that is, some atoms easily give up electrons, while others tend to accept them (usually these are atoms of the elements that form typical metals and atoms of elements forming typical nonmetals); the electronegativity of such atoms is also very different.
Ionic bonding is non-directional and non-saturable.
Covalent bond- a chemical bond that occurs due to the formation of a common pair of electrons. A covalent bond is formed between small atoms with the same or similar radii. A necessary condition is the presence of unpaired electrons in both bonded atoms ( exchange mechanism) or a lone pair on one atom and a free orbital on the other (donor-acceptor mechanism):
| A) | H· + ·H H:H | H-H | H 2 | (one shared pair of electrons; H is monovalent); |
| b) | NN | N 2 | (three shared pairs of electrons; N is trivalent); | |
| V) | H-F | HF | (one shared pair of electrons; H and F are monovalent); | |
| G) | NH4+ | (four shared pairs of electrons; N is tetravalent) |
- Based on the number of shared electron pairs, covalent bonds are divided into
- simple (single)- one pair of electrons,
- double- two pairs of electrons,
- triples- three pairs of electrons.
Double and triple bonds are called multiple bonds.
According to the distribution of electron density between bonded atoms covalent bond divided by non-polar And polar. A non-polar bond is formed between identical atoms, a polar one - between different ones.
Electronegativity- a measure of the ability of an atom in a substance to attract common electron pairs.
The electron pairs of polar bonds are shifted towards more electronegative elements. The displacement of electron pairs itself is called bond polarization. The partial (excess) charges formed during polarization are designated + and -, for example: .
Based on the nature of the overlap of electron clouds ("orbitals"), a covalent bond is divided into -bond and -bond.
-A bond is formed due to the direct overlap of electron clouds (along the straight line connecting the atomic nuclei), -a bond is formed due to lateral overlap (on both sides of the plane in which the atomic nuclei lie).
A covalent bond is directional and saturable, as well as polarizable.
The hybridization model is used to explain and predict the mutual direction of covalent bonds.
Hybridization of atomic orbitals and electron clouds- the supposed alignment of atomic orbitals in energy, and electron clouds in shape when an atom forms covalent bonds.
The three most common types of hybridization are: sp-, sp 2 and sp 3 -hybridization. For example:
sp-hybridization - in molecules C 2 H 2, BeH 2, CO 2 (linear structure);
sp 2-hybridization - in molecules C 2 H 4, C 6 H 6, BF 3 (flat triangular shape);
sp 3-hybridization - in molecules CCl 4, SiH 4, CH 4 (tetrahedral form); NH 3 (pyramidal shape); H 2 O (angular shape).
Metal connection- a chemical bond formed by sharing the valence electrons of all bonded atoms of a metal crystal. As a result, a single electron cloud of the crystal is formed, which easily moves under the influence of electrical voltage - hence the high electrical conductivity of metals.
A metallic bond forms when the atoms being bonded are large and therefore tend to give up electrons. Simple substances with a metallic bond are metals (Na, Ba, Al, Cu, Au, etc.), complex substances are intermetallic compounds (AlCr 2, Ca 2 Cu, Cu 5 Zn 8, etc.).
The metal bond does not have directionality or saturation. It is also preserved in metal melts.
Hydrogen bond- an intermolecular bond formed due to the partial acceptance of a pair of electrons from a highly electronegative atom by a hydrogen atom with a large positive partial charge. It is formed in cases where one molecule contains an atom with a lone pair of electrons and high electronegativity (F, O, N), and the other contains a hydrogen atom bound by a highly polar bond to one of such atoms. Examples of intermolecular hydrogen bonds:
H—O—H OH 2 , H—O—H NH 3 , H—O—H F—H, H—F H—F.
Intramolecular hydrogen bonds exist in polypeptide molecules, nucleic acids, proteins, etc.
A measure of the strength of any bond is the bond energy.
Communication energy- the energy required to break a given chemical bond in 1 mole of a substance. The unit of measurement is 1 kJ/mol.
The energies of ionic and covalent bonds are of the same order, the energy of hydrogen bonds is an order of magnitude less.
The energy of a covalent bond depends on the size of the bonded atoms (bond length) and on the multiplicity of the bond. The smaller the atoms and the greater the bond multiplicity, the greater its energy.
The ionic bond energy depends on the size of the ions and their charges. The smaller the ions and the greater their charge, the greater the binding energy.
Structure of matter
According to the type of structure, all substances are divided into molecular And non-molecular. Among organic substances, molecular substances predominate, among inorganic substances, non-molecular substances predominate.
Based on the type of chemical bond, substances are divided into substances with covalent bonds, substances with ionic bonds (ionic substances) and substances with metallic bonds (metals).
Substances with covalent bonds can be molecular or non-molecular. This significantly affects their physical properties.
Molecular substances consist of molecules connected to each other by weak intermolecular bonds, these include: H 2, O 2, N 2, Cl 2, Br 2, S 8, P 4 and others simple substances; CO 2, SO 2, N 2 O 5, H 2 O, HCl, HF, NH 3, CH 4, C 2 H 5 OH, organic polymers and many other substances. These substances do not have high strength, they have low temperatures melting and boiling, do not carry out electric current, some of them are soluble in water or other solvents.
Non-molecular substances with covalent bonds or atomic substances (diamond, graphite, Si, SiO 2, SiC and others) form very strong crystals (with the exception of layered graphite), they are insoluble in water and other solvents, have high melting and boiling points, most of them they do not conduct electric current (except for graphite, which is electrically conductive, and semiconductors - silicon, germanium, etc.)
All ionic substances are naturally non-molecular. These are solid, refractory substances, solutions and melts of which conduct electric current. Many of them are soluble in water. It should be noted that in ionic substances ah, the crystals of which consist of complex ions, there are also covalent bonds, for example: (Na +) 2 (SO 4 2-), (K +) 3 (PO 4 3-), (NH 4 +)(NO 3-) etc. The atoms that make up complex ions are connected by covalent bonds.
Metals (substances with metallic bonds) very diverse in their physical properties. Among them there are liquid (Hg), very soft (Na, K) and very hard metals (W, Nb).
Characteristic physical properties metals are their high electrical conductivity (unlike semiconductors, it decreases with increasing temperature), high heat capacity and ductility (for pure metals).
In the solid state, almost all substances are composed of crystals. Based on the type of structure and type of chemical bond, crystals (“crystal lattices”) are divided into atomic(crystals of non-molecular substances with covalent bonds), ionic(crystals of ionic substances), molecular(crystals of molecular substances with covalent bonds) and metal(crystals of substances with a metallic bond).
Tasks and tests on the topic "Topic 10. "Chemical bonding. Structure of matter."
- Types of chemical bond - Structure of matter grade 8–9
Lessons: 2 Assignments: 9 Tests: 1
- Assignments: 9 Tests: 1
Having worked through this topic, you should understand the following concepts: chemical bond, intermolecular bond, ionic bond, covalent bond, metallic bond, hydrogen bond, single bond, double bond, triple bond, multiple bonds, non-polar bond, polar bond, electronegativity, bond polarization, - and - bond, hybridization of atomic orbitals, bond energy.
You must know the classification of substances by type of structure, by type of chemical bond, the dependence of the properties of simple and complex substances on the type of chemical bond and the type of “crystal lattice”.
You must be able to: determine the type of chemical bond in a substance, the type of hybridization, draw up diagrams of bond formation, use the concept of electronegativity, a number of electronegativity; know how electronegativity changes chemical elements one period, and one group to determine the polarity of a covalent bond.
After making sure that everything you need has been learned, proceed to completing the tasks. We wish you success.
Recommended reading:
- O. S. Gabrielyan, G. G. Lysova. Chemistry 11th grade. M., Bustard, 2002.
- G. E. Rudzitis, F. G. Feldman. Chemistry 11th grade. M., Education, 2001.
Option 2
Part A:
A 1. A pair of elements between which an ionic chemical bond is formed:
a) carbon and sulfur, b) hydrogen and nitrogen, c) potassium and oxygen, d) silicon and hydrogen.
A 2.Formula of a substance with a covalent bond:
a) NaCl, b) HCl, c) BaO, d) Ca 3 N 2.
A 3.The least polar bond is:
a) C – H, b) C – Cl, c) C – F, d) C – Br.
A 4. The statement that δ is a bond, in contrast to π, is true:
a) less durable, b) formed when atomic orbitals overlap laterally,
c) is not covalent, d) is formed by the axial overlap of atomic orbitals.
A 5.A substance in the molecule of which there is no π bond:
a) ethylene, b) benzene, c) ammonia, d) nitrogen.
A 6. The strongest molecule is:
a) H 2, b) N 2, c) F 2, d) O 2.
A 7. In the CO 3 2- ion, the carbon atom is in the sp 2 - hybrid state, so the ion has the form:
a) linear, b) tetrahedron, c) triangle, d) octahedron.
A 8. A carbon atom has an oxidation number of -3 and a valency of 4 when combined with the formula:
a) CO 2, b) C 2 H 6, c) CH 3 Cl, d) CaC 2.
A 9. The atomic crystal lattice has:
a) soda, b) water, c) diamond, d) paraffin.
A 10. A substance between molecules of which there is a hydrogen bond:
a) ethane, b) sodium fluoride, c) carbon monoxide (4), d) ethanol.
A 11. Select a group of elements arranged in order of increasing electronegativity:
a) Cl, Si, N, O, b) Si, P, N, F, c) F, Cl, O, Si, d) O, N, F, Cl.
A 12. There is a covalent bond between atoms, formed by a donor-acceptor mechanism in a substance, the formula of which is:
13.
A 14.The formation of hydrogen bonds can be explained by:
a) solubility acetic acid in water, b) acid properties ethanol,
c) high melting point of many metals, d) insolubility of methane in water.
A 15.Formula of a substance with a polar covalent bond:
a) Cl 2, b) KCl, c) NH 3, d) O 2.
Part B:
B 1. From among those proposed, select a substance whose molecule contains π bonds: H 2, CH 4, Br 2, N 2, H 2 S, CH 3 OH, NH 3. Write the name of this substance.
B 2. Interaction process electron orbitals, leading to their alignment in shape and energy, is called......
B 3. What is the name of the phenomenon of enlargement of colloidal particles and their precipitation from a colloidal solution?
B 4. Give an example of a substance whose molecule contains three δ – and one π – bonds. Name the substance in the nominative case.
B 5. In which of the following substances are the bonds most polar: hydrogen chloride, fluorine, water, ammonia, hydrogen sulfide. Write down the selected substance with a formula.
Part C:
From 1. Write structural formulas all isomeric substances of the composition C 4 H 8. Name each substance.
C 2. Make up the structural formulas of the substances: CHF 3, C 2 H 2 Br 2, O 2.
Compose graphic formulas: Mg 3 N 2, Na 2 SO 4, KHCO 3.
C 3.
Mg 3 N 2, Cl 2, ZnSO 4, KHS, CH 3 Cl, FeOHCl 2, BrO 2, AsO 4 3-, NH 4 +
Test No. 2 “STRUCTURE OF MATTER”.
Option 3
Part A:
A 1. Chemical bonds in substances whose formulas are CH 4 and CaCl 2, respectively:
a) ionic and covalent polar, b) covalent polar and ionic,
c) covalent non-polar and ionic, d) covalent polar and metallic.
A 2.The polarity of the bond is greater in the substance with the formula:
a) Br 2, b) LiBr, c) HBr, d) KBr
A 3.The ionic nature of the bond in the series of compounds Li 2 O - Na 2 O - K 2 O - Rb 2 O:
a) increases, b) decreases, c) does not change, d) first decreases, then increases.
A 4. There is a covalent bond between atoms, formed by a donor-acceptor mechanism in a substance, the formula of which is:
a) Al(OH) 3, b) [CH 3 NH 3 ]Cl, c) C 2 H 5 OH, d) C 6 H 12 O 6.
A 5.A couple of formulas for substances whose molecules contain only δ bonds:
a) CH 4 and O 2, b) C 2 H 5 OH and H 2 O, c) N 2 and CO 2, d) HBr and C 2 H 4.
A 6. The strongest connection of these:
a) C - Cl, b) C - F, c) C - Br, d) C - I.
A 7. A group of formulas of compounds in which there is a similar direction of bonds, due to sp 3 - hybridization of electronic orbitals:
a) CH 4, C 2 H 4, C 2 H 2, b) NH 3, CH 4, H 2 O, c) H 2 O, C 2 H 6, C 6 H 6, d) C 3 H 8, BCl 3, BeCl 2.
A 8. The valency and oxidation state of the carbon atom in the methanol molecule are respectively equal to:
a) 4 and +4, b) 4 and -2, c) 3 and +2, d) 4 and -3.
A 9. Substances with ionic crystal lattice characterized by:
a) poor solubility in water, b) high boiling point, c) fusibility, d) volatility.
A 10. The formation of a hydrogen bond between molecules leads to:
a) to reduce the boiling points of substances, b) to reduce the solubility of substances in water,
c) to an increase in the boiling points of substances, d) to an increase in the volatility of substances.
A 11. Formula of a substance with an ionic bond:
a) NH 3, b) C 2 H 4, c) KH, d) CCl 4.
A 12. Only δ – bond is present in the molecule:
a) nitrogen, b) ethanol, c) ethylene, d) carbon monoxide (4).
13. The molecular structure has a substance with the formula:
a) CH 4, b) NaOH, c) SiO 2, d) Al.
A 14.A hydrogen bond is formed between:
a) water molecules, b) hydrogen molecules,
c) hydrocarbon molecules, d) metal atoms and hydrogen atoms.
A 15.If you vigorously shake the mixture of vegetable oil and water, you get:
a) suspension, b) emulsion, c) foam, d) aerosol.
Part B:
B 1. The number of common electron pairs between bromine atoms in a Br 2 molecule is……
B 2. What bonds form the triple bond in molecule N 2 (present your answer in the nominative case).
B 3. At the nodes of the metal crystal lattice there are…….. .
B 4. Give an example of a substance whose molecule contains five δ - and two π - bonds. Name the substance in the nominative case.
B 5. What is the maximum number of π bonds that can form between two atoms in a molecule? (represent the answer as a number)
Part C:
From 1. Write the structural formulas of all isomeric substances of the composition C 5 H 10 O. Name each substance.
C 2. Make up the structural formulas of the substances: CHCl 3, C 2 H 2 Cl 2, F 2.
Make up graphic formulas: AlN, CaSO 4, LiHCO 3.
C 3. Determine the oxidation state in chemical compounds and ions:
HNO 3, HClO 4, K 2 SO 3, KMnO 4, CH 3 F, MgOHCl 2, ClO 3 -, CrO 4 2-, NH 4 +
Related information.
Test No. 2 DKR “STRUCTURE OF SUBSTANCE”.
A 1. Chemical bonds in substances whose formulas are CH 4 and CaCl 2, respectively:
a) ionic and covalent polar, b) covalent polar and ionic,
c) covalent non-polar and ionic, d) covalent polar and metallic.
A 2. The polarity of the bond is greater in the substance with the formula:
a) Br 2, b) LiBr, c) HBr, d) KBr
A 3. The ionic nature of the bond in the series of compounds Li 2 O - Na 2 O - K 2 O - Rb 2 O:
a) increases, b) decreases, c) does not change, d) first decreases, then increases.
A 4. There is a covalent bond between atoms, formed by a donor-acceptor mechanism in a substance, the formula of which is:
a) Al(OH) 3, b) [CH 3 NH 3 ]Cl, c) C 2 H 5 OH, d) C 6 H 12 O 6.
A 5. A couple of formulas for substances whose molecules contain only δ bonds:
a) CH 4 and O 2, b) C 2 H 5 OH and H 2 O, c) N 2 and CO 2, d) HBr and C 2 H 4.
A 6. The strongest connection of these:
a) C - Cl, b) C - F, c) C - Br, d) C - I.
A 7. A group of formulas of compounds in which there is a similar direction of bonds, due to sp 3 - hybridization of electronic orbitals:
a) CH 4, C 2 H 4, C 2 H 2, b) NH 3, CH 4, H 2 O, c) H 2 O, C 2 H 6, C 6 H 6, d) C 3 H 8, BCl 3, BeCl 2.
A 8. The valency and oxidation state of the carbon atom in the methanol molecule are respectively equal to:
a) 4 and +4, b) 4 and -2, c) 3 and +2, d) 4 and -3.
A 9. Substances with an ionic crystal lattice are characterized by:
a) poor solubility in water, b) high boiling point, c) fusibility, d) volatility.
A 10. The formation of a hydrogen bond between molecules leads to:
a) to reduce the boiling points of substances, b) to reduce the solubility of substances in water,
c) to an increase in the boiling points of substances, d) to an increase in the volatility of substances.
A 11. Formula of a substance with an ionic bond:
a) NH 3, b) C 2 H 4, c) KH, d) CCl 4.
A 12
A13. The molecular structure has a substance with the formula:
A 14. A hydrogen bond is formed between:
a) water molecules, b) hydrogen molecules,
c) hydrocarbon molecules, d) metal atoms and hydrogen atoms.
A 15. If you vigorously shake the mixture of vegetable oil and water, you get:
a) suspension, b) emulsion, c) foam, d) aerosol.
A 16. Formula of a substance with a polar covalent bond:
a) Cl 2, b) KCl, c) NH 3, d) O 2.
A 17. A substance between the molecules of which there is a hydrogen bond:
a) ethanol, b) methane, c) Hydrogen, d) Benzene.
A 18. Number of shared electron pairs in a hydrogen molecule:
a) one, b) two, c) three, d) four.
A 19. The polarity of a chemical bond increases in a number of compounds whose formulas are:
a) NH 3, HI, O 2, b) CH 4, H 2 O, HF, c) PH 3, H 2 S, H 2, d) HCl, CH 4, CL 2.
A 20. Crystal lattice of sodium chloride:
a) atomic, b) ionic, c) metallic, d) molecular.
A 21. Number of δ and π bonds in an acetylene molecule:
a) 5 δ and π - no, b) 2 δ and 3 π, c) 3 δ and 2 π, d) 4 δ and 1 π.
A 22. Substances whose formulas are: CH 3 – CH 2 – OH and CH 3 – O – CH 3 are:
a) homologues, b) isomers, c) the same substance, d) both homologues and isomers.
A 23. The homolog of a substance whose formula is CH 2 = CH – CH 3 is:
a) butane, b) butene - 1, c) butene - 2, d) butine - 1.
A 24. A covalent nonpolar bond is formed between atoms:
a) hydrogen and oxygen, b) carbon and hydrogen, c) chlorine, d) magnesium.
A 25. Only δ – bond is present in the molecule:
a) nitrogen, b) ethanol, c) ethylene, d) carbon monoxide (4).
A 26. The nitrogen atom has a valence of 3 and an oxidation state of 0 in a molecule of a substance whose formula is:
a) NH 3, b) N 2, c) CH 3 NO 2, d) N 2 O 3.
A 27. The molecular structure has a substance with the formula:
a) CH 4, b) NaOH, c) SiO 2, d) Al.
A28. The C–H bond is stronger than the Si–H bond because:
a) the bond length is shorter, b) the bond length is longer,
c) the polarity of the bond is less, d) the polarity of the bond is greater.
A 29. There is a covalent bond between atoms, formed by a donor-acceptor mechanism in a substance, the formula of which is:
a) CH 3 NO 2, b) NH 4 NO 2, c) C 5 H 8, d) H 2 O.
A 30. The least polar bond is:
a) C – H, b) C – Cl, c) C – F, d) C – Br
Part B:
B 1. The number of common electron pairs between bromine atoms in a Br 2 molecule is……
B 2. What bonds form the triple bond in molecule N 2 (present your answer in the nominative case).
B 3. At the nodes of the metal crystal lattice there are…….. .
B 4. Give an example of a substance whose molecule contains five δ - and two π - bonds. Name the substance in the nominative case.
B 5.
B 6. The number of common electron pairs between bromine atoms in the N 2 molecule is……
B 7. What bonds form the triple bond in the C 2 H 2 molecule (present your answer in the nominative case).
B 8. At the nodes of the ionic crystal lattice there are........ .
B 9. Give an example of a substance whose molecule contains five δ – and one π – bonds. Name the substance in the nominative case.
B 10. What is the maximum number of π bonds that can form between two atoms in a molecule? (represent the answer as a number)
Part C:
From 1. Write the structural formulas of all isomeric substances of the composition C 5 H 10 O. Name each substance.
C 2 .
Make up the structural formulas of the substances: CHCl 3, C 2 H 2 Cl 2, F 2.
Make up graphic formulas: AlN, CaSO 4, LiHCO 3.
C 3.
HNO 3, HClO 4, K 2 SO 3, KMnO 4, CH 3 F, MgOHCl 2, ClO 3 -, CrO 4 2-, NH 4 +
C 4. Write the structural formulas of all isomeric substances of the composition C 4 H 8 O 2. Name each substance.
C 5 .
Make up the structural formulas of the substances: CHBr 3, C 2 H 2 Br 2, Br 2.
Make up graphic formulas: Al 2 S 3, MgSO 4, Li 2 CO 3.
From 6. Determine the degree of oxidation in chemical compounds and ions:
CCl 4, Ba(NO 3) 2, Al 2 S 3, HClO 3, Na 2 Cr 2 O 7, K 2 O 4, SrO 2-, Cr 2 O 3 2
page 1
Novikova Olesya Vladimirovna
Chemistry and Biology Teacher
MOU - secondary school s. Prokudino
Atkarsky district
Saratov region.
Test No. 1 on the topic: “Structure of substances.”
Option I .
a) hydrogen chloride
b) sodium hydroxide
c) carbon monoxide (II)
d) carbon monoxide (IV)
2. A polar covalent bond is present in the molecule
a) oxygen
b) rhombic sulfur
d) hydrogen
3. Chemical bond in a carbon dioxide molecule
a) covalent nonpolar
b) covalent polar
c) metal
d) ionic
4. The strongest molecule is:
A) H 2 ;
b) N 2 ;
V) F 2 ;
G) O 2 .
5. A substance between the molecules of which there is a hydrogen bond:
b) sodium fluoride;
c) carbon monoxide (II);
d) ethanol.
6. Substances with an ionic crystal lattice are characterized by:
a) poor solubility in water; c) fusibility;
b) high boiling point; d) volatility.
7. The formation of a hydrogen bond between molecules leads to:
a) to a decrease in boiling points;
b) to reduce the solubility of substances in water;
c) to increase boiling temperatures;
d) to increase the volatility of substances.
8. Which substance contains more oxygen in Na 2 CO 3 or in Ca(HCO 3 ) 2?
9. :
A) SO 2 +H 2 O͢͢→
B) Na+H 2 O→
B) Na 2 O+H 2 O→
D) S+H 2 O→
10. Solve the problem :
How much water and sodium hydroxide are needed to prepare 180 g of a 15% solution?
11 . Solve the problem :
What is the mass of oxygen obtained by fractional distillation of 200 m 3 (n.s.) air, if the volume fraction of oxygen is 0.21?
Test No. 1 on the topic “Structure of substances.”
Option II .
Ionic chemical bonding occurs in
a) crystalline sulfur
b) solid iodine
c) calcium iodide
d) phosphorus oxide (v)
2. A covalent polar bond is present in the molecule
a) sulfuric acid
b) plastic sulfur
d) rubidium sulfide
3. Chemical bond in a hydrogen molecule
a) covalent nonpolar
b) covalent polar
c) metal
d) ionic
4. The strongest bonds in a molecule of a substance whose formula is:
A) H 2 S ;
b) H 2 Se ;
V) H 2 O ;
G) H 2 Te .
5. The molecular structure has substances with the formula:
A) CH 4 ;
b) NaOH ;
V) SiO 2 ;
G) Al .
6. A hydrogen bond is formed between:
a) water molecules; c) hydrocarbon molecules;
b) hydrogen molecules; d) metal atoms and hydrogen atoms.
7. The formation of hydrogen bonds can be explained by:
a) solubility of acetic acid in water;
b) acidic properties of ethanol;
c) high melting point of many metals;
d) insolubility of methane in water.
8. Compare the sulfur content of Mg(HSO 4) 2 and CuSO 4?
9. Complete the equations of possible reactions :
A) CO 2 +H 2 O͢͢→
B) Al+H 2 O→
B) Fe+H 2 O→
D) C+H 2 O→
10. Solve the problem:
It is necessary to prepare 540 g of 12% solution nitric acid. Calculate how much water and acid to take to prepare such a solution.
11. Solve the problem:
What is the mass of nitrogen obtained from 143.6 liters of air containing 78% nitrogen by volume?