In biology, ATP is the source of energy and the basis of life. ATP - adenosine triphosphate - is involved in metabolic processes and regulates biochemical reactions in the body.
What's this?
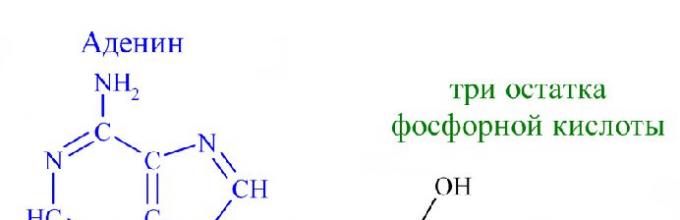
To understand what ATP is, chemistry will help. Chemical formula ATP molecules - C10H16N5O13P3. Remembering the full name is easy if you break it down into its component parts. Adenosine triphosphate or adenosine triphosphoric acid is a nucleotide consisting of three parts:
- adenine - purine nitrogenous base;
- ribose - monosaccharide related to pentoses;
- three residues phosphoric acid.

Rice. 1. The structure of the ATP molecule.
A more detailed breakdown of ATP is presented in the table.
ATP was first discovered by Harvard biochemists Subbarao, Loman, and Fiske in 1929. In 1941, the German biochemist Fritz Lipmann established that ATP is the energy source of a living organism.
Energy generation
Phosphate groups are interconnected by high-energy bonds that are easily destroyed. During hydrolysis (interaction with water), the bonds of the phosphate group break down, releasing a large number of energy, and ATP is converted to ADP (adenosine diphosphoric acid).
Conventionally, the chemical reaction looks like this:
TOP 4 articleswho read along with this
ATP + H2O → ADP + H3PO4 + energy

Rice. 2. Hydrolysis of ATP.
Part of the released energy (about 40 kJ / mol) is involved in anabolism (assimilation, plastic metabolism), part is dissipated in the form of heat to maintain body temperature. With further hydrolysis of ADP, another phosphate group is cleaved off with the release of energy and the formation of AMP (adenosine monophosphate). AMP does not undergo hydrolysis.
ATP synthesis
ATP is located in the cytoplasm, nucleus, chloroplasts, and mitochondria. Synthesis of ATP in animal cage occurs in mitochondria, and in plant - in mitochondria and chloroplasts.
ATP is formed from ADP and phosphate with the expenditure of energy. This process is called phosphorylation:
ADP + H3PO4 + energy → ATP + H2O

Rice. 3. Formation of ATP from ADP.
In plant cells, phosphorylation occurs during photosynthesis and is called photophosphorylation. In animals, the process occurs during respiration and is called oxidative phosphorylation.
In animal cells, ATP synthesis occurs in the process of catabolism (dissimilation, energy metabolism) during the breakdown of proteins, fats, carbohydrates.
Functions
From the definition of ATP, it is clear that this molecule is capable of providing energy. In addition to energy, adenosine triphosphoric acid performs other features:
- is a material for the synthesis of nucleic acids;
- is part of enzymes and regulates chemical processes, speeding up or slowing down their course;
- is a mediator - transmits a signal to synapses (points of contact of two cell membranes).
19. Functions of cell mitochondria. ATP and its role in the cell.
The main source of energy for the cell are nutrients: carbohydrates, fats and proteins, which are oxidized with the help of oxygen. Almost all carbohydrates, before reaching the cells of the body, are converted into glucose due to the work of the gastrointestinal tract and liver. Along with carbohydrates, proteins are also broken down - to amino acids and lipids - to fatty acids. In the cell, nutrients are oxidized under the influence of oxygen and with the participation of enzymes that control the reactions of energy release and its utilization. Almost all oxidative reactions occur in the mitochondria, and the released energy is stored in the form of a macroergic compound - ATP. In the future, it is ATP, and not nutrients, that is used to provide energy for intracellular metabolic processes.
The ATP molecule contains: (1) the nitrogenous base adenine; (2) pentose carbohydrate ribose, (3) three phosphoric acid residues. The last two phosphates are connected to each other and to the rest of the molecule by macroergic phosphate bonds, indicated by the symbol ~ in the ATP formula. Subject to the physical and chemical conditions characteristic of the body, the energy of each such bond is 12,000 calories per 1 mol of ATP, which is many times higher than the energy of an ordinary chemical bond, which is why phosphate bonds are called macroergic. Moreover, these bonds are easily destroyed, providing intracellular processes with energy as soon as the need arises.
When energy is released, ATP donates a phosphate group and turns into adenosine diphosphate. The released energy is used for almost all cellular processes, for example, in biosynthesis reactions and during muscle contraction.
Replenishment of ATP reserves occurs by recombining ADP with the rest of phosphoric acid due to the energy of nutrients. This process is repeated over and over again. ATP is constantly consumed and accumulated, which is why it is called the energy currency of the cell. The turnover time of ATP is only a few minutes.
The role of mitochondria in chemical reactions formation of ATP. When glucose enters the cell, under the action of cytoplasmic enzymes it turns into pyruvic acid (this process is called glycolysis). The energy released in this process is used to convert a small amount of ADP to ATP, less than 5% of the total energy reserves.
ATP synthesis is 95% carried out in mitochondria. Pyruvic acid, fatty acids and amino acids, formed respectively from carbohydrates, fats and proteins, are eventually converted in the mitochondrial matrix into a compound called acetyl-CoA. This compound, in turn, enters into a series of enzymatic reactions, collectively known as the tricarboxylic acid cycle or the Krebs cycle, to give up its energy. In the tricarboxylic acid cycle, acetyl-CoA is broken down into hydrogen atoms and carbon dioxide molecules. Carbon dioxide is removed from the mitochondria, then from the cell by diffusion and excreted from the body through the lungs.
Hydrogen atoms are chemically very active and therefore immediately react with oxygen diffusing into the mitochondria. The large amount of energy released in this reaction is used to convert many ADP molecules into ATP. These reactions are quite complex and require the participation of a huge number of enzymes that make up the mitochondrial cristae. On the initial stage an electron is split off from a hydrogen atom, and the atom becomes a hydrogen ion. The process ends with the addition of hydrogen ions to oxygen. As a result of this reaction, water and a large amount of energy are formed that are necessary for the operation of ATP synthetase, a large globular protein that acts as tubercles on the surface of mitochondrial cristae. Under the action of this enzyme, which uses the energy of hydrogen ions, ADP is converted into ATP. New ATP molecules are sent from the mitochondria to all parts of the cell, including the nucleus, where the energy of this compound is used to provide a variety of functions. This process ATP synthesis is generally called the chemiosmotic mechanism of ATP formation.
Millions of biochemical reactions take place in any cell of our body. They are catalyzed by a variety of enzymes that often require energy. Where does the cell take it? This question can be answered if we consider the structure of the ATP molecule - one of the main sources of energy.
ATP is a universal source of energy
ATP stands for adenosine triphosphate, or adenosine triphosphate. Matter is one of the two most important sources of energy in any cell. The structure of ATP and biological role closely connected. Most biochemical reactions can only take place with the participation of molecules of a substance, especially this applies. However, ATP is rarely directly involved in the reaction: for any process to take place, energy is needed that is contained precisely in adenosine triphosphate.
The structure of the molecules of the substance is such that the bonds formed between the phosphate groups carry a huge amount of energy. Therefore, such bonds are also called macroergic, or macroenergetic (macro=many, large number). The term was first introduced by the scientist F. Lipman, and he also suggested using the icon ̴ to designate them.
It is very important for the cell to maintain a constant level of adenosine triphosphate. This is especially true for muscle cells and nerve fibers, because they are the most energy-dependent and need a high content of adenosine triphosphate to perform their functions.
The structure of the ATP molecule
Adenosine triphosphate is made up of three elements: ribose, adenine, and

Ribose- a carbohydrate that belongs to the group of pentoses. This means that ribose contains 5 carbon atoms, which are enclosed in a cycle. Ribose is connected to adenine by a β-N-glycosidic bond on the 1st carbon atom. Also, phosphoric acid residues on the 5th carbon atom are attached to the pentose.
Adenine is a nitrogenous base. Depending on which nitrogenous base is attached to the ribose, GTP (guanosine triphosphate), TTP (thymidine triphosphate), CTP (cytidine triphosphate) and UTP (uridine triphosphate) are also isolated. All these substances are similar in structure to adenosine triphosphate and perform approximately the same functions, but they are much less common in the cell.
Residues of phosphoric acid. A maximum of three phosphoric acid residues can be attached to a ribose. If there are two or only one of them, then, respectively, the substance is called ADP (diphosphate) or AMP (monophosphate). It is between the phosphorus residues that macroenergetic bonds are concluded, after the rupture of which from 40 to 60 kJ of energy is released. If two bonds are broken, 80, less often - 120 kJ of energy is released. When the bond between the ribose and the phosphorus residue is broken, only 13.8 kJ is released, therefore, there are only two high-energy bonds in the triphosphate molecule (P ̴ P ̴ P), and one in the ADP molecule (P ̴ P).
What are the structural features of ATP. Due to the fact that a macroenergetic bond is formed between phosphoric acid residues, the structure and functions of ATP are interconnected.
The structure of ATP and the biological role of the molecule. Additional functions of adenosine triphosphate
In addition to energy, ATP can perform many other functions in the cell. Along with other nucleotide triphosphates, triphosphate is involved in the construction nucleic acid. In this case, ATP, GTP, TTP, CTP and UTP are the suppliers of nitrogenous bases. This property is used in processes and transcription.
ATP is also required for the operation of ion channels. For example, the Na-K channel pumps 3 molecules of sodium out of the cell and pumps 2 molecules of potassium into the cell. Such an ion current is needed to maintain a positive charge on the outer surface of the membrane, and only with the help of adenosine triphosphate can the channel function. The same applies to proton and calcium channels.
ATP is a precursor of the second messenger cAMP (cyclic adenosine monophosphate) - cAMP not only transmits the signal received by the cell membrane receptors, but is also an allosteric effector. Allosteric effectors are substances that speed up or slow down enzymatic reactions. So, cyclic adenosine triphosphate inhibits the synthesis of an enzyme that catalyzes the breakdown of lactose in bacterial cells.
The adenosine triphosphate molecule itself can also be an allosteric effector. Moreover, in such processes, ADP acts as an ATP antagonist: if triphosphate accelerates the reaction, then diphosphate slows down, and vice versa. These are the functions and structure of ATP.

How is ATP formed in the cell
The functions and structure of ATP are such that the molecules of the substance are quickly used and destroyed. Therefore, the synthesis of triphosphate is an important process in the formation of energy in the cell.
There are three most important ways to synthesize adenosine triphosphate:
1. Substrate phosphorylation.
2. Oxidative phosphorylation.
3. Photophosphorylation.
Substrate phosphorylation is based on multiple reactions occurring in the cytoplasm of the cell. These reactions are called glycolysis - the anaerobic stage. As a result of 1 glycolysis cycle, two molecules are synthesized from 1 glucose molecule, which are further used for energy production, and two ATP are also synthesized.
- C 6 H 12 O 6 + 2ADP + 2Fn --> 2C 3 H 4 O 3 + 2ATP + 4H.
Cell respiration
Oxidative phosphorylation is the formation of adenosine triphosphate by the transfer of electrons along the electron transport chain of the membrane. As a result of this transfer, a proton gradient is formed on one of the sides of the membrane, and with the help of the protein integral set of ATP synthase, molecules are built. The process takes place on the mitochondrial membrane.

The sequence of steps of glycolysis and oxidative phosphorylation in mitochondria makes up the overall process called respiration. After a complete cycle, 36 ATP molecules are formed from 1 glucose molecule in the cell.
Photophosphorylation
The process of photophosphorylation is the same oxidative phosphorylation with only one difference: photophosphorylation reactions occur in the chloroplasts of the cell under the action of light. ATP is produced during the light stage of photosynthesis, the main energy-producing process in green plants, algae, and some bacteria.
In the process of photosynthesis, electrons pass through the same electron transport chain, resulting in the formation of a proton gradient. The concentration of protons on one side of the membrane is the source of ATP synthesis. The assembly of molecules is carried out by the enzyme ATP synthase.

The average cell contains 0.04% adenosine triphosphate of the total mass. However, the most great importance observed in muscle cells: 0.2-0.5%.
There are about 1 billion ATP molecules in a cell.
Each molecule lives no more than 1 minute.
One molecule of adenosine triphosphate is renewed 2000-3000 times a day.
In total, the human body synthesizes 40 kg of adenosine triphosphate per day, and at each time point the supply of ATP is 250 g.

Conclusion
The structure of ATP and the biological role of its molecules are closely related. The substance plays a key role in life processes, because the macroergic bonds between phosphate residues contain a huge amount of energy. Adenosine triphosphate performs many functions in the cell, and therefore it is important to maintain a constant concentration of the substance. Decay and synthesis proceed at a high speed, since the energy of bonds is constantly used in biochemical reactions. It is an indispensable substance of any cell of the body. That, perhaps, is all that can be said about the structure of ATP.
Any organism can exist as long as there is an intake of nutrients from the external environment and as long as the products of its vital activity are released into this environment. Inside the cell there is a continuous very complex complex of chemical transformations, due to which the components of the cell body are formed from nutrients. The totality of the processes of transformation of matter in a living organism, accompanied by its constant renewal, is called metabolism.
Part of the overall metabolism, which consists in the absorption, assimilation of nutrients and the creation at their expense structural components cells, is called assimilation - this is a constructive exchange. The second part of the general exchange is the processes of dissimilation, i.e. decomposition and oxidation processes organic matter, as a result of which the cell receives energy, is an energy exchange. Constructive and energy exchange constitute a single whole.
In the process of constructive exchange, a cell synthesizes biopolymers of its body from a rather limited number of low molecular weight compounds. Biosynthetic reactions proceed with the participation of various enzymes and require energy.
Living organisms can only use chemically bound energy. Each substance has a certain reserve potential energy. Its main material carriers are chemical bonds, the rupture or transformation of which leads to the release of energy. Energy level Some bonds have a value of 8-10 kJ - these bonds are called normal. Other bonds contain much more energy - 25-40 kJ - these are the so-called macroergic bonds. Almost all known compounds with such bonds have phosphorus or sulfur atoms in their composition, in the place of which these bonds are localized in the molecule. Adenosine triphosphoric acid (ATP) is one of the compounds that play an important role in cell life.
Adenosine triphosphoric acid (ATP) consists of an organic adenine base (I), a ribose carbohydrate (II) and three phosphoric acid residues (III). The combination of adenine and ribose is called adenosine. Pyrophosphate groups have macroergic bonds, indicated by ~. The decomposition of one ATP molecule with the participation of water is accompanied by the elimination of one molecule of phosphoric acid and the release of free energy, which is 33-42 kJ / mol. All reactions involving ATP are regulated by enzyme systems.
Fig.1. Adenosine triphosphoric acid (ATP)
Energy metabolism in the cell. ATP synthesis
ATP synthesis occurs in mitochondrial membranes during respiration, therefore all enzymes and cofactors of the respiratory chain, all enzymes of oxidative phosphorylation are localized in these organelles.
ATP synthesis occurs in such a way that two H + ions are split off from ADP and phosphate (P) on the right side of the membrane, compensating for the loss of two H + during the reduction of substance B. One of the oxygen atoms of the phosphate is transferred to the other side of the membrane and, having attached two H ions + from the left compartment, forms H 2 O. The phosphoryl residue attaches to ADP, forming ATP.

Fig.2. Scheme of ATP oxidation and synthesis in mitochondrial membranes
In the cells of organisms, many biosynthetic reactions have been studied that use the energy contained in ATP, during which the processes of carboxylation and decarboxylation, the synthesis of amide bonds, the formation of macroergic compounds capable of transferring energy from ATP to anabolic reactions of synthesis of substances occur. These reactions play an important role in the metabolic processes of plant organisms.
With the participation of ATP and other high-energy nucleoside polyphosphates (GTP, CTP, UGF), monosaccharide molecules, amino acids, nitrogenous bases, acylglycerols can be activated by the synthesis of active intermediates that are derivatives of nucleotides. So, for example, in the process of starch synthesis with the participation of the enzyme ADP-glucose pyrophosphorylase, an activated form of glucose is formed - adenosine diphosphate glucose, which easily becomes a donor of glucose residues during the formation of the structure of the molecules of this polysaccharide.
ATP synthesis occurs in the cells of all organisms in the process of phosphorylation, i.e. addition of inorganic phosphate to ADP. The energy for ADP phosphorylation is generated during energy metabolism. Energy metabolism, or dissimilation, is a set of splitting reactions of organic substances, accompanied by the release of energy. Depending on the habitat, dissimilation can proceed in two or three stages.
In most living organisms - aerobes living in an oxygen environment - three stages are carried out during dissimilation: preparatory, oxygen-free and oxygen, during which organic substances decompose to inorganic compounds. In anaerobes living in an environment devoid of oxygen, or in aerobes with a lack of it, dissimilation occurs only in the first two stages with the formation of intermediate organic compounds still rich in energy.
The first stage - preparatory - consists in the enzymatic splitting of complex organic compounds into simpler ones (proteins - into amino acids, fats - into glycerol and fatty acids, polysaccharides - into monosaccharides, nucleic acids - into nucleotides). The breakdown of organic food substrates is carried out at different levels of the gastrointestinal tract of multicellular organisms. Intracellular cleavage of organic substances occurs under the action of hydrolytic enzymes of lysosomes. The energy released in this case is dissipated in the form of heat, and the resulting small organic molecules can undergo further splitting or be used by the cell as a “building material” for the synthesis of its own organic compounds.
The second stage - incomplete oxidation (oxygen-free) - is carried out directly in the cytoplasm of the cell, it does not need the presence of oxygen and consists in further splitting of organic substrates. The main source of energy in the cell is glucose. Anoxic, incomplete breakdown of glucose is called glycolysis.
Glycolysis is a multi-stage enzymatic process of converting six-carbon glucose into two three-carbon molecules of pyruvic acid (pyruvate, PVA) C3H4O3. During the reactions of glycolysis, a large amount of energy is released - 200 kJ / mol. Part of this energy (60%) is dissipated as heat, the rest (40%) is used for ATP synthesis.
As a result of glycolysis of one glucose molecule, two molecules of PVC, ATP and water are formed, as well as hydrogen atoms, which are stored by the cell in the form of NADH, i.e. as part of a specific carrier - nicotinamide adenine dinucleotide. The further fate of glycolysis products - pyruvate and hydrogen in the form of NAD H - can develop in different ways. In yeast or in plant cells, when there is a lack of oxygen, alcoholic fermentation- PVC is reduced to ethyl alcohol:
In animal cells experiencing a temporary lack of oxygen, for example, in human muscle cells during excessive exercise, as well as in some bacteria, lactic acid fermentation occurs, in which pyruvate is reduced to lactic acid. In the presence of oxygen in the environment, the products of glycolysis undergo further splitting to final products.
The third stage - complete oxidation (respiration) - proceeds with the obligatory participation of oxygen. Aerobic respiration is a chain of reactions controlled by enzymes of the inner membrane and mitochondrial matrix. Once in the mitochondria, PVC interacts with matrix enzymes and forms: carbon dioxide, which is excreted from the cell; hydrogen atoms, which, as part of the carriers, are sent to the inner membrane; acetyl coenzyme A (acetyl-CoA), which is involved in the tricarboxylic acid cycle (Krebs cycle). The Krebs cycle is a chain of successive reactions during which two CO2 molecules, an ATP molecule and four pairs of hydrogen atoms are formed from one acetyl-CoA molecule, transferred to carrier molecules - NAD and FAD (flavin adenine dinucleotide). The overall reaction of glycolysis and the Krebs cycle can be represented as follows:
So, as a result of the oxygen-free stage of dissimilation and the Krebs cycle, the glucose molecule is broken down to inorganic carbon dioxide (CO2), and the energy released in this process is partially spent on ATP synthesis, but is mainly saved in the electron-loaded carriers NAD H2 and FAD H2. Carrier proteins transport hydrogen atoms to the inner mitochondrial membrane, where they are passed along a chain of proteins built into the membrane. The transport of particles along the transport chain is carried out in such a way that protons remain on the outer side of the membrane and accumulate in the intermembrane space, turning it into an H+ reservoir, while electrons are transferred to the inner surface of the inner mitochondrial membrane, where they eventually combine with oxygen.
As a result of the activity of the electron transport chain enzymes, the inner mitochondrial membrane is negatively charged from the inside, and positively charged from the outside (due to H), so that a potential difference is created between its surfaces. It is known that molecules of the enzyme ATP synthetase with an ion channel are embedded in the inner membrane of mitochondria. When the potential difference across the membrane reaches a critical level (200 mV), the positively charged H+ particles begin to push through the ATPase channel by the force of the electric field and, once on the inner surface of the membrane, interact with oxygen, forming water.
The normal course of metabolic reactions at the molecular level is due to the harmonious combination of the processes of catabolism and anabolism. When catabolic processes are disturbed, first of all, energy difficulties arise, ATP regeneration is disrupted, as well as the supply of the initial anabolism substrates necessary for biosynthetic processes. In turn, damage to anabolic processes that is primary or associated with changes in catabolism processes leads to a disruption in the reproduction of functionally important compounds - enzymes, hormones, etc.
Violation of various links of metabolic chains is unequal in its consequences. The most significant, profound pathological changes in catabolism occur when the biological oxidation system is damaged due to blockade of tissue respiration enzymes, hypoxia, etc., or damage to the mechanisms of conjugation of tissue respiration and oxidative phosphorylation (for example, uncoupling of tissue respiration and oxidative phosphorylation in thyrotoxicosis). In these cases, the cells are deprived of the main source of energy, almost all oxidative reactions catabolism are blocked or lose the ability to accumulate the released energy in ATP molecules. By inhibiting the reactions of the tricarboxylic acid cycle, energy production from catabolism is reduced by about two-thirds.
Adenosine triphosphoric acid - ATP
Nucleotides are the structural basis for a number of organic substances important for life, for example, macroergic compounds.
ATP is the universal source of energy in all cells. adenosine triphosphoric acid or adenosine triphosphate.
ATP is found in the cytoplasm, mitochondria, plastids and cell nuclei and is the most common and universal source of energy for most biochemical reactions occurring in the cell.
ATP provides energy for all cell functions: mechanical work, biosynthesis of substances, fission, etc. On average, the ATP content in a cell is about 0.05% of its mass, but in those cells where ATP costs are high (for example, in liver cells, striated muscles), its content can reach up to 0.5%.
The structure of ATP
ATP is a nucleotide consisting of a nitrogenous base - adenine, a ribose carbohydrate and three phosphoric acid residues, two of which store a large amount of energy.
The bond between phosphoric acid residues is called macroergic(it is denoted by the symbol ~ ), since when it breaks, almost 4 times more energy is released than when other chemical bonds are split.
ATP is an unstable structure and when separating one residue of phosphoric acid, ATP passes into adenosine diphosphate (ADP) releasing 40 kJ of energy.
Other nucleotide derivatives
Hydrogen carriers constitute a special group of nucleotide derivatives. Molecular and atomic hydrogen has a high chemical activity and is released or absorbed during various biochemical processes. One of the most widely used hydrogen carriers is nicotinamide dinucleotide phosphate(NADP).
The NADP molecule is capable of attaching two atoms or one molecule of free hydrogen, turning into a reduced form NADP H 2
. In this form, hydrogen can be used in various biochemical reactions.
Nucleotides can also take part in the regulation of oxidative processes in the cell.
vitamins
Vitamins (from lat. vita- life) - complex bioorganic compounds, absolutely necessary in small quantities for the normal functioning of living organisms. Vitamins differ from other organic substances in that they are not used as a source of energy or building material. Some vitamins organisms can synthesize themselves (for example, bacteria are able to synthesize almost all vitamins), other vitamins enter the body with food.
Vitamins are usually denoted by letters of the Latin alphabet. The modern classification of vitamins is based on their ability to dissolve in water and fats (they are divided into two groups: water soluble(B 1 , B 2 , B 5 , B 6 , B 12 , PP , C) and fat-soluble(A , D , E , K)).
Vitamins are involved in almost all biochemical and physiological processes that together make up the metabolism. Both deficiency and excess of vitamins can lead to serious impairment of many physiological functions in the body.