Physical properties
Benzene and its closest homologues are colorless liquids with a specific odor. Aromatic hydrocarbons are lighter than water and do not dissolve in it, but they easily dissolve in organic solvents - alcohol, ether, acetone.
Benzene and its homologues are themselves good solvents for many organic matter... All arenas burn with a smoky flame due to the high carbon content in their molecules.
The physical properties of some arenas are presented in the table.
Table. Physical properties of some arenas
|
Name |
Formula |
t ° .pl., |
t °. boil., |
|
Benzene |
C 6 H 6 |
5,5 |
80,1 |
|
Toluene (methylbenzene) |
C 6 H 5 CH 3 |
95,0 |
110,6 |
|
Ethylbenzene |
C 6 H 5 C 2 H 5 |
95,0 |
136,2 |
|
Xylene (dimethylbenzene) |
C 6 H 4 (CH 3) 2 |
||
|
ortho- |
25,18 |
144,41 |
|
|
meta- |
47,87 |
139,10 |
|
|
couple- |
13,26 |
138,35 |
|
|
Propyl benzene |
C 6 H 5 (CH 2) 2 CH 3 |
99,0 |
159,20 |
|
Cumene (isopropylbenzene) |
C 6 H 5 CH (CH 3) 2 |
96,0 |
152,39 |
|
Styrene (vinyl benzene) |
C 6 H 5 CH \u003d CH 2 |
30,6 |
145,2 |
Benzene - low-boiling ( t bale\u003d 80.1 ° C), colorless liquid, insoluble in water
Attention! Benzene - poison, acts on the kidneys, changes the blood formula (with prolonged exposure), can disrupt the structure of chromosomes.
Most aromatic hydrocarbons are life-threatening and toxic.
Obtaining arenes (benzene and its homologues)
In the laboratory
1. Fusion of salts of benzoic acid with solid alkalis
C 6 H 5 -COONa + NaOH t →C 6 H 6 + Na 2 CO 3
sodium benzoate
2. Würz-Fitting reaction: (here G is halogen)
C 6H 5 -G + 2Na + R-G →C 6 H 5 - R + 2 NaD
FROM 6 H 5 -Cl + 2Na + CH 3 -Cl → C 6 H 5 -CH 3 + 2NaCl
In industry
- isolated from oil and coal by fractional distillation, reforming;
- from coal tar and coke oven gas
1. Dehydrocyclization of alkanes with more than 6 carbon atoms:
C 6 H 14 t , kat→ C 6 H 6 + 4H 2
2. Acetylene trimerization (for benzene only) - r. Zelinsky:
3C 2 H 2 600 ° C , Act. coal → C 6 H 6
3. Dehydrogenation cyclohexane and its homologues:
Soviet Academician Nikolai Dmitrievich Zelinsky established that benzene is formed from cyclohexane (dehydrogenation of cycloalkanes
C 6 H 12 t, kat→ C 6 H 6 + 3H 2

C 6 H 11 -CH 3 t , kat→ C 6 H 5 -CH 3 + 3H 2
methylcyclohexantholuene
4. Alkylation of benzene (obtaining homologues of benzene) - p Friedel-Crafts.
C 6 H 6 + C 2 H 5 -Cl t, AlCl3→ C 6 H 5 -C 2 H 5 + HCl
chloroethane ethylbenzene

Chemical properties of arenes
I... OXIDATION REACTIONS
1. Combustion (smoky flame):
2C 6 H 6 + 15O 2 t → 12CO 2 + 6H 2 O + Q
2. Benzene under normal conditions does not discolor bromine water and an aqueous solution of potassium permanganate
3. Homologues of benzene are oxidized by potassium permanganate (decolorize potassium permanganate):
A) in an acidic medium to benzoic acid
Under the action of potassium permanganate and other strong oxidants on homologues of benzene, the side chains are oxidized. No matter how complex the substituent chain is, it is destroyed, with the exception of the a-carbon atom, which is oxidized to a carboxyl group.
Homologues of benzene with one side chain give benzoic acid:
Homologues containing two side chains give dibasic acids:
5C 6 H 5 -C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 → 5C 6 H 5 COOH + 5CO 2 + 6K 2 SO 4 + 12MnSO 4 + 28H 2 O
5C 6 H 5 -CH 3 + 6KMnO 4 + 9H 2 SO 4 → 5C 6 H 5 COOH + 3K 2 SO 4 + 6MnSO 4 + 14H 2 O
Simplified :
C 6 H 5 -CH 3 + 3O KMnO4→ C 6 H 5 COOH + H 2 O
B) in neutral and slightly alkaline to benzoic acid salts
C 6 H 5 -CH 3 + 2KMnO 4 → C 6 H 5 COOК + K ОН + 2MnO 2 + H 2 O
II... ADDITIONAL REACTIONS (harder than alkenes)
1. Halogenation
C 6 H 6 + 3Cl 2 h ν → C 6 H 6 Cl 6 (hexachlorocyclohexane - hexachloran)
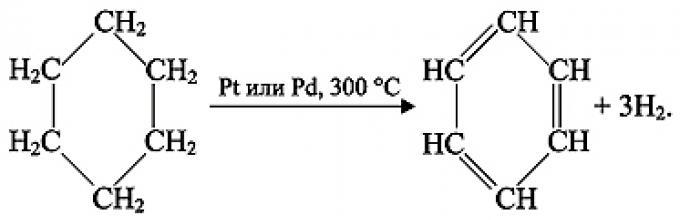
2. Hydrogenation
C 6 H 6 + 3H 2 t , Pt or Ni → C 6 H 12 (cyclohexane)
3. Polymerization
III. SUBSTITUTION REACTIONS - ionic mechanism (lighter than alkanes)
1. Halogenation -
a ) benzene
C 6 H 6 + Cl 2 AlCl 3 → C 6 H 5 -Cl + HCl (chlorobenzene)
C 6 H 6 + 6Cl 2 t, AlCl3→ C 6 Cl 6 + 6HCl( hexachlorobenzene)
C 6 H 6 + Br 2 t, FeCl3→ C 6 H 5 -Br + HBr( bromobenzene)
b) homologues of benzene upon irradiation or heating
In terms of chemical properties, alkyl radicals are similar to alkanes. Hydrogen atoms in them are replaced by halogen by a free radical mechanism. Therefore, in the absence of a catalyst upon heating or UV irradiation, a radical substitution reaction occurs in the side chain. The effect of the benzene ring on alkyl substituents leads to the fact that the hydrogen atom at the carbon atom directly bonded to the benzene ring (a-carbon atom) is always replaced.
1) C 6 H 5 -CH 3 + Cl 2 h ν → C 6 H 5 -CH 2 -Cl + HCl
c) homologues of benzene in the presence of a catalyst
C 6 H 5 -CH 3 + Cl 2 AlCl 3 → (mixture of ort, a pair of derivatives) + HCl
2. Nitration (with nitric acid)
C 6 H 6 + HO-NO 2 t, H2SO4→ C 6 H 5 -NO 2 + H 2 O
nitrobenzene - smell almonds!
C 6 H 5 -CH 3 + 3HO-NO 2 t, H2SO4→ FROM H 3 -C 6 H 2 (NO 2) 3 + 3H 2 O2,4,6-trinitrotoluene (tol, TNT)
The use of benzene and its homologues
Benzene C 6 H 6 is a good solvent. Benzene as an additive improves the quality of motor fuel. Serves as a raw material for many aromatic organic compounds - nitrobenzene C 6 H 5 NO 2 (solvent, aniline is obtained from it), chlorobenzene C 6 H 5 Cl, phenol C 6 H 5 OH, styrene, etc.
Toluene C 6 H 5 –CH 3 is a solvent used in the production of dyes, drugs and explosives (TNT (tol), or 2,4,6-trinitrotoluene TNT).
XylenesC 6 H 4 (CH 3) 2. Technical xylene is a mixture of three isomers ( ortho-, meta- and couple-xylenes) - is used as a solvent and a starting product for the synthesis of many organic compounds.
Isopropylbenzene C 6 H 5 –CH (CH 3) 2 is used to obtain phenol and acetone.
Chlorine derivatives of benzene used for plant protection. So, the product of substitution of H atoms in benzene by chlorine atoms is hexachlorobenzene С 6 Сl 6 - fungicide; it is used for dry dressing of wheat and rye seeds against hard smut. The product of addition of chlorine to benzene - hexachlorocyclohexane (hexachloran) C 6 H 6 Cl 6 - insecticide; it is used to control harmful insects. The mentioned substances belong to pesticides - chemical agents for fighting microorganisms, plants and animals.
Styrene C 6 H 5 - CH \u003d CH 2 polymerizes very easily, forming polystyrene, and copolymerizing with butadiene - styrene butadiene rubbers.
VIDEO EXPERIENCES
In redox reactions, organic substances more often exhibit the properties of reducing agents, and themselves are oxidized. The ease of oxidation of organic compounds depends on the availability of electrons when interacting with an oxidizing agent. All known factors causing an increase in the electron density in molecules of organic compounds (for example, positive inductive and mesomeric effects) will increase their ability to oxidize and vice versa.
The tendency of organic compounds to oxidize increases with the growth of their nucleophilicity, which corresponds to the following rows:
Increase in nucleophilicity in a row
Consider redox reactions members of the most important classes organic matter with some inorganic oxidizing agents.
Alkenes oxidation
Mild oxidation converts alkenes to glycols (dihydric alcohols). Reducing atoms in these reactions are carbon atoms linked by a double bond.
The reaction with a solution of potassium permanganate proceeds in a neutral or weakly alkaline medium as follows:
3C 2 H 4 + 2KMnO 4 + 4H 2 O → 3CH 2 OH – CH 2 OH + 2MnO 2 + 2KOH
Under more severe conditions, oxidation leads to the breaking of the carbon chain at the double bond and the formation of two acids (in a strongly alkaline medium - two salts) or an acid and carbon dioxide (in a strongly alkaline medium - salt and carbonate):
1) 5CH 3 CH \u003d CHCH 2 CH 3 + 8KMnO 4 + 12H 2 SO 4 → 5CH 3 COOH + 5C 2 H 5 COOH + 8MnSO 4 + 4K 2 SO 4 + 17H 2 O
2) 5CH 3 CH \u003d CH 2 + 10KMnO 4 + 15H 2 SO 4 → 5CH 3 COOH + 5CO 2 + 10MnSO 4 + 5K 2 SO 4 + 20H 2 O
3) CH 3 CH \u003d CHCH 2 CH 3 + 8KMnO 4 + 10KOH → CH 3 COOK + C 2 H 5 COOK + 6H 2 O + 8K 2 MnO 4
4) CH 3 CH \u003d CH 2 + 10KMnO 4 + 13KOH → CH 3 COOK + K 2 CO 3 + 8H 2 O + 10K 2 MnO 4
Potassium dichromate in a sulfuric acid medium oxidizes alkenes similarly to reactions 1 and 2.
During the oxidation of alkenes, in which the carbon atoms at the double bond contain two carbon radicals, two ketones are formed:


Oxidation of alkynes
Alkines oxidize under somewhat more severe conditions than alkenes, so they usually oxidize with a triple bond breaking of the carbon chain. As in the case of alkenes, the reducing atoms here are carbon atoms linked by multiple bonds. The reactions produce acids and carbon dioxide. Oxidation can be carried out with potassium permanganate or dichromate in an acidic medium, for example:
5CH 3 C≡CH + 8KMnO 4 + 12H 2 SO 4 → 5CH 3 COOH + 5CO 2 + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O

Acetylene can be oxidized with potassium permanganate in a neutral medium to potassium oxalate:
3CH≡CH + 8KMnO 4 → 3KOOC –COOK + 8MnO 2 + 2KON + 2H 2 O
In an acidic environment, oxidation proceeds to oxalic acid or carbon dioxide:
5CH≡CH + 8KMnO 4 + 12H 2 SO 4 → 5HOOC –COOH + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O
CH≡CH + 2KMnO 4 + 3H 2 SO 4 → 2CO 2 + 2MnSO 4 + 4H 2 O + K 2 SO 4
Oxidation of benzene homologues
Benzene does not oxidize even under rather harsh conditions. Homologues of benzene can be oxidized with a solution of potassium permanganate in a neutral medium to potassium benzoate:
C 6 H 5 CH 3 + 2KMnO 4 → C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O
C 6 H 5 CH 2 CH 3 + 4KMnO 4 → C 6 H 5 COOK + K 2 CO 3 + 2H 2 O + 4MnO 2 + KOH
Oxidation of benzene homologues with potassium dichromate or permanganate in an acidic medium leads to the formation of benzoic acid.
5C 6 H 5 CH 3 + 6KMnO 4 +9 H 2 SO 4 → 5C 6 H 5 COOH + 6MnSO 4 + 3K 2 SO 4 + 14H 2 O
5C 6 H 5 –C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 → 5C 6 H 5 COOH + 5CO 2 + 12MnSO 4 + 6K 2 SO 4 + 28H 2 O


Oxidation of alcohols
The direct oxidation product of primary alcohols is aldehydes, and of secondary alcohols, ketones.
The aldehydes formed during the oxidation of alcohols are easily oxidized to acids; therefore, aldehydes from primary alcohols are obtained by oxidation with potassium dichromate in an acidic medium at the boiling point of the aldehyde. Evaporating, aldehydes do not have time to oxidize.
3C 2 H 5 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O
With an excess of an oxidizing agent (KMnO 4, K 2 Cr 2 O 7) in any medium, primary alcohols are oxidized to carboxylic acids or their salts, and the secondary ones - to ketones.
5C 2 H 5 OH + 4KMnO 4 + 6H 2 SO 4 → 5CH 3 COOH + 4MnSO 4 + 2K 2 SO 4 + 11H 2 O
3CH 3 –CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 → 3CH 3 –COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O
Tertiary alcohols are not oxidized under these conditions, and methyl alcohol is oxidized to carbon dioxide.

Dihydric alcohol, ethylene glycol HOCH 2 –CH 2 OH, when heated in an acidic medium with a solution of KMnO 4 or K 2 Cr 2 O 7 is easily oxidized to oxalic acid, and in neutral to potassium oxalate.
5СН 2 (ОН) - СН 2 (ОН) + 8КMnO 4 + 12H 2 SO 4 → 5HOOC –COOH + 8MnSO 4 + 4K 2 SO 4 + 22Н 2 О
3СН 2 (ОН) - СН 2 (ОН) + 8КMnO 4 → 3KOOC –COOK + 8MnO 2 + 2КОН + 8Н 2 О
Oxidation of aldehydes and ketones
Aldehydes are quite strong reducing agents, and therefore are easily oxidized by various oxidizing agents, for example: KMnO 4, K 2 Cr 2 O 7, OH, Cu (OH) 2. All reactions take place when heated:
3CH 3 CHO + 2KMnO 4 → CH 3 COOH + 2CH 3 COOK + 2MnO 2 + H 2 O
3CH 3 CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 COOH + Cr 2 (SO 4) 3 + 7H 2 O
CH 3 CHO + 2KMnO 4 + 3KOH → CH 3 COOK + 2K 2 MnO 4 + 2H 2 O
5CH 3 CHO + 2KMnO 4 + 3H 2 SO 4 → 5CH 3 COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O
CH 3 CHO + Br 2 + 3NaOH → CH 3 COONa + 2NaBr + 2H 2 O

silver mirror reaction
With an ammonia solution of silver oxide, aldehydes are oxidized to carboxylic acids, which in an ammonia solution give ammonium salts (reaction of the "silver mirror"):
CH 3 CH \u003d O + 2OH → CH 3 COONH 4 + 2Ag + H 2 O + 3NH 3
CH 3 –CH \u003d O + 2Cu (OH) 2 → CH 3 COOH + Cu 2 O + 2H 2 O
Formic aldehyde (formaldehyde) is usually oxidized to carbon dioxide:
5HCOH + 4KMnO 4 (hut) + 6H 2 SO 4 → 4MnSO 4 + 2K 2 SO 4 + 5CO 2 + 11H 2 O
3СН 2 О + 2K 2 Cr 2 O 7 + 8H 2 SO 4 → 3CO 2 + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O
HCHO + 4OH → (NH 4) 2 CO 3 + 4Ag ↓ + 2H 2 O + 6NH 3
HCOH + 4Cu (OH) 2 → CO 2 + 2Cu 2 O ↓ + 5H 2 O
Ketones are oxidized under harsh conditions by strong oxidizing agents with rupture c-C links and give a mixture of acids:

Carboxylic acids.Among acids, formic and oxalic have strong reducing properties, which are oxidized to carbon dioxide.
HCOOH + HgCl 2 \u003d CO 2 + Hg + 2HCl
HCOOH + Cl 2 \u003d CO 2 + 2HCl
HOOC-COOH + Cl 2 \u003d 2CO 2 + 2HCl
Formic acid, Besides acidic properties, also exhibits some properties of aldehydes, in particular, reducing. In doing so, it is oxidized to carbon dioxide. For instance:
2KMnO4 + 5HCOOH + 3H2SO4 → K2SO4 + 2MnSO4 + 5CO2 + 8H2O
When heated with strong dehydrating agents (H2SO4 (conc.) Or P4O10) decomposes:
HCOOH → (t) CO + H2O
Catalytic oxidation of alkanes:

Catalytic oxidation of alkenes:

Oxidation of phenols:


18. Redox reactions (continued 2)
18.9. OVR with the participation of organic substances
In OVR organic substances with inorganic organic substances are most often reducing agents. So, when organic matter is burned in an excess of oxygen, carbon dioxide and water are always formed. The reactions are more complicated when less active oxidants are used. In this section, only the reactions of representatives of the most important classes of organic substances with some inorganic oxidants are considered.
Alkenes. Mild oxidation converts alkenes to glycols (dihydric alcohols). Reducing atoms in these reactions are carbon atoms linked by a double bond.
The reaction with a solution of potassium permanganate proceeds in a neutral or weakly alkaline medium as follows:
C 2 H 4 + 2KMnO 4 + 2H 2 O CH 2 OH – CH 2 OH + 2MnO 2 + 2KOH (cooling)
Under more severe conditions, oxidation leads to the breaking of the carbon chain at the double bond and the formation of two acids (in a strongly alkaline medium - two salts) or an acid and carbon dioxide (in a strongly alkaline medium - salt and carbonate):
1) 5CH 3 CH \u003d CHCH 2 CH 3 + 8KMnO 4 + 12H 2 SO 4 5CH 3 COOH + 5C 2 H 5 COOH + 8MnSO 4 + 4K 2 SO 4 + 17H 2 O (heating)
2) 5CH 3 CH \u003d CH 2 + 10KMnO 4 + 15H 2 SO 4 5CH 3 COOH + 5CO 2 + 10MnSO 4 + 5K 2 SO 4 + 20H 2 O (heating)
3) CH 3 CH \u003d CHCH 2 CH 3 + 6KMnO 4 + 10KOH CH 3 COOK + C 2 H 5 COOK + 6H 2 O + 6K 2 MnO 4 (heating)
4) CH 3 CH \u003d CH 2 + 10KMnO 4 + 13KOH CH 3 COOK + K 2 CO 3 + 8H 2 O + 10K 2 MnO 4 (heating)
Potassium dichromate in a sulfuric acid medium oxidizes alkenes similarly to reactions 1 and 2.
Alkyne. Alkines begin to oxidize under somewhat harsher conditions than alkenes, so they usually oxidize with a triple bond breaking of the carbon chain. As in the case of alkanes, the reducing atoms here are carbon atoms linked in this case by a triple bond. The reactions produce acids and carbon dioxide. Oxidation can be carried out with potassium permanganate or dichromate in an acidic medium, for example:
5CH 3 C CH + 8KMnO 4 + 12H 2 SO 4 5CH 3 COOH + 5CO 2 + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O (heating)
Sometimes it is possible to isolate intermediate oxidation products. Depending on the position of the triple bond in the molecule, these are either diketones (R 1 –CO – CO – R 2) or aldoketones (R – CO – CHO).
Acetylene can be oxidized with potassium permanganate in a slightly alkaline medium to potassium oxalate:
3C 2 H 2 + 8KMnO 4 \u003d 3K 2 C 2 O 4 + 2H 2 O + 8MnO 2 + 2KOH
In an acidic environment, oxidation proceeds to carbon dioxide:
C 2 H 2 + 2KMnO 4 + 3H 2 SO 4 \u003d 2CO 2 + 2MnSO 4 + 4H 2 O + K 2 SO 4
Homologues of benzene. Homologues of benzene can be oxidized with a solution of potassium permanganate in a neutral medium to potassium benzoate:
C 6 H 5 CH 3 + 2KMnO 4 \u003d C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O (when boiling)
C 6 H 5 CH 2 CH 3 + 4KMnO 4 \u003d C 6 H 5 COOK + K 2 CO 3 + 2H 2 O + 4MnO 2 + KOH (when heated)
Oxidation of these substances with potassium dichromate or permanganate in an acidic medium leads to the formation of benzoic acid.
Alcohols. The direct oxidation product of primary alcohols is aldehydes, and of secondary alcohols, ketones.
The aldehydes formed during the oxidation of alcohols are easily oxidized to acids; therefore, aldehydes from primary alcohols are obtained by oxidation with potassium dichromate in an acidic medium at the boiling point of the aldehyde. Evaporating, aldehydes do not have time to oxidize.
3C 2 H 5 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O (heating)
With an excess of an oxidizing agent (KMnO 4, K 2 Cr 2 O 7) in any medium, primary alcohols are oxidized to carboxylic acids or their salts, and secondary alcohols - to ketones. Tertiary alcohols are not oxidized under these conditions, and methyl alcohol is oxidized to carbon dioxide. All reactions take place when heated.
Dihydric alcohol, ethylene glycol HOCH 2 –CH 2 OH, when heated in an acidic medium with a solution of KMnO 4 or K 2 Cr 2 O 7 is easily oxidized to carbon dioxide and water, but sometimes intermediate products (HOCH 2 –COOH, HOOC– COOH, etc.).
Aldehydes. Aldehydes are quite strong reducing agents, and therefore are easily oxidized by various oxidizing agents, for example: KMnO 4, K 2 Cr 2 O 7, OH. All reactions take place when heated:
3CH 3 CHO + 2KMnO 4 \u003d CH 3 COOH + 2CH 3 COOK + 2MnO 2 + H 2 O
3CH 3 CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 COOH + Cr 2 (SO 4) 3 + 7H 2 O
CH 3 CHO + 2OH \u003d CH 3 COONH 4 + 2Ag + H 2 O + 3NH 3
Formaldehyde with an excess of an oxidizing agent is oxidized to carbon dioxide.
18.10. Comparison of the redox activity of various substances
From the definitions of the concepts "oxidizing atom" and "reducing agent" it follows that atoms in the highest oxidation state have only oxidizing properties. On the contrary, atoms in the lowest oxidation state have only reducing properties. Atoms in intermediate oxidation states can be both oxidizing and reducing agents.
At the same time, based only on the degree of oxidation, it is impossible to unambiguously assess the redox properties of substances. As an example, consider the compounds of group VA elements. Compounds of nitrogen (V) and antimony (V) are more or less strong oxidants, bismuth (V) compounds are very strong oxidizers, and phosphorus (V) compounds practically do not possess oxidizing properties. In this and other similar cases, it matters how much a given oxidation state is characteristic of a given element, that is, how stable the compounds containing atoms of a given element in this oxidation state are.
Any ORP flows in the direction of the formation of a weaker oxidizing agent and a weaker reducing agent. In the general case, the possibility of any ORR, like any other reaction, can be determined by the sign of the change in the Gibbs energy. In addition, the electrochemical characteristics of oxidizing agents and reducing agents (standard potentials of redox pairs) are used to quantify the redox activity of substances. Based on these quantitative characteristics, it is possible to construct series of redox activity of various substances. The series of metal stresses known to you is constructed in exactly this way. This series makes it possible to compare the reducing properties of metals in aqueous solutions under standard conditions ( from \u003d 1 mol / l, T \u003d 298.15 K), as well as the oxidizing properties of simple aquacations. If ions (oxidizers) are placed in the top line of this row, and metal atoms (reducing agents) are placed in the bottom row, then the left side of this row (before hydrogen) will look like this:
In this row, the oxidizing properties of ions (top row) increase from left to right, and the reducing properties of metals (bottom row), on the contrary, from right to left.
Taking into account the differences in redox activity in different media, it is possible to construct similar series for oxidants. Thus, for reactions in an acidic medium (pH \u003d 0), a "continuation" of a series of metal activities in the direction of enhancing oxidizing properties is obtained
As in the row of metal activities, in this row the oxidizing properties of oxidants (top row) increase from left to right. But, using this series, it is possible to compare the reducing activity of the reducing agents (bottom line) only if their oxidized form coincides with that given in the top line; in this case, it is amplified from right to left.
Let's look at some examples. To find out whether this ORR is possible, we will use the general rule that determines the direction of the redox reactions (the reactions proceed in the direction of the formation of a weaker oxidizing agent and a weaker reducing agent).
1. Is it possible to recover cobalt with magnesium from a CoSO 4 solution?
Magnesium is a stronger reducing agent than cobalt, and Co 2 ions are stronger oxidizing agents than Mg 2 ions, therefore, it is possible.
2. Is it possible to oxidize copper to CuCl 2 with a FeCl 3 solution in an acidic environment?
Since Fe 3B ions are stronger oxidants than Cu 2 ions, and copper is a stronger reducing agent than Fe 2 ions, it is possible.
3. Is it possible, by blowing oxygen through a FeCl 2 solution acidified with hydrochloric acid, to obtain a FeCl 3 solution?
It would seem not, since in our series oxygen is to the left of the Fe 3 ions and is a weaker oxidant than these ions. But in aqueous solution oxygen is practically never reduced to H 2 O 2, in which case it is reduced to H 2 O and takes a place between Br 2 and MnO 2. Therefore, such a reaction is possible, however, it proceeds rather slowly (why?).
4. Is it possible to oxidize H 2 O 2 in an acidic environment with potassium permanganate?
In this case, H 2 O 2 reducing agent and reducing agent are stronger than Mn 2B ions, and MnO 4 ions are oxidizing agents stronger than oxygen formed from peroxide. Therefore, it is possible.
A similar series built for ORP in an alkaline medium is as follows:
Unlike the "acid" series, this series cannot be used in conjunction with the metal activity range.
The method of electron-ion balance (method of half-reactions), intermolecular ORR, intramolecular ORR, ORR dismutation (disproportionation, self-oxidation-self-healing), ORR contouring, passivation.
- Using the method of electron-ion balance, compose the equations of the reactions occurring when a solution of a) H 2 S (S, more precisely, S 8) is added to a solution of potassium permanganate acidified with sulfuric acid; b) KHS; c) K 2 S; d) H 2 SO 3; e) KHSO 3; f) K 2 SO 3; e) HNO 2; g) KNO 2; i) KI (I 2); j) FeSO 4; k) C 2 H 5 OH (CH 3 COOH); m) CH 3 CHO; m) (COOH) 2 (CO 2); n) K 2 C 2 O 4. Hereinafter, where necessary, the oxidation products are indicated in curly brackets.
- Make up the equations of the reactions proceeding when passing the following gases through a solution of potassium permanganate acidified with sulfuric acid: a) C 2 H 2 (CO 2); b) C 2 H 4 (CO 2); c) C 3 H 4 (propyne) (CO 2 and CH 3 COOH); d) C 3 H 6; e) CH 4; f) HCHO.
- The same, but a solution of a reducing agent is added to a neutral solution of potassium permanganate: a) KHS; b) K 2 S; c) KHSO 3; d) K 2 SO 3; e) KNO 2; f) KI.
- The same, but a solution of potassium hydroxide was previously added to the potassium permanganate solution: a) K 2 S (K 2 SO 4); b) K 2 SO 3; c) KNO 2; d) KI (KIO 3).
- Make up the equations for the following reactions in solution: a) KMnO 4 + H 2 S ...;
b) KMnO 4 + HCl ...;
c) KMnO 4 + HBr ...;
d) KMnO 4 + HI ... - Make the following equations for the ORP of manganese dioxide:
- To acidified with sulfuric acid solution of potassium dichromate added solutions of the following substances: a) KHS; b) K 2 S; c) HNO 2; d) KNO 2; e) KI; f) FeSO 4; g) CH 3 CH 2 CHO; i) H 2 SO 3; j) KHSO 3; k) K 2 SO 3. Make up the equations of the ongoing reactions.
- The same, but the following gases were passed through the solution: a) H 2 S; b) SO 2.
- To a solution of potassium chromate containing potassium hydroxide, added solutions a) K 2 S (K 2 SO 4); b) K 2 SO 3; c) KNO 2; d) KI (KIO 3). Make up the equations of the ongoing reactions.
- Potassium hydroxide solution was added to the chromium (III) chloride solution until the initially formed precipitate was dissolved, and then bromine water was added. Make up the equations of the ongoing reactions.
- The same, but at the last stage a solution of potassium peroxodisulfate K 2 S 2 O 8 was added, which was reduced during the reaction to sulfate.
- Make the equations of the reactions taking place in the solution:
- Make up the equations for the reactions between solid chromium trioxide and the following substances: a) C; b) CO; c) S (SO 2); d) H 2 S; e) NH 3; f) C 2 H 5 OH (CO 2 and H 2 O); g) CH 3 COCH 3.
- Make up the equations of the reactions that occur when the following substances are added to concentrated nitric acid: a) S (H 2 SO 4); b) P 4 ((HPO 3) 4); c) graphite; d) Se; e) I 2 (HIO 3); f) Ag; g) Cu; i) Pb; j) KF; k) FeO; m) FeS; m) MgO; n) MgS; p) Fe (OH) 2; c) P 2 O 3; m) As 2 O 3 (H 3 AsO 4); y) As 2 S 3; t) Fe (NO 3) 2; x) P 4 O 10; c) Cu 2 S.
- The same, but when passing the following gases: a) CO; b) H 2 S; c) N 2 O; d) NH 3; e) NO; f) H 2 Se; g) HI.
- The reactions will proceed in the same or different way in the following cases: a) a piece of magnesium is placed in a high test tube two-thirds filled with concentrated nitric acid; b) a drop of concentrated nitric acid was placed on the surface of the magnesium plate? Write the reaction equations.
- What is the difference between the reaction of concentrated nitric acid with hydrogen sulfide acid and with gaseous hydrogen sulfide? Write the reaction equations.
- Will ORP proceed the same when anhydrous crystalline sodium sulfide and its 0.1 M solution are added to a concentrated solution of nitric acid?
- A mixture of the following substances was treated with concentrated nitric acid: Cu, Fe, Zn, Si and Cr. Make up the equations of the ongoing reactions.
- Make up the equations of the reactions that occur when the following substances are added to dilute nitric acid: a) I 2; b) Mg; c) Al; d) Fe; e) FeO; f) FeS; g) Fe (OH) 2; i) Fe (OH) 3; j) MnS; l) Cu 2 S; m) CuS; m) CuO; n) Na 2 S cr; p) Na 2 S p; c) P 4 O 10.
- What processes will occur when passing through a dilute solution of nitric acid a) ammonia, b) hydrogen sulfide, c) carbon dioxide?
- Write down the equations for the reactions that occur when added to concentrated sulfuric acid the following substances: a) Ag; b) Cu; c) graphite; d) HCOOH; e) C 6 H 12 O 6; f) NaCl cr; g) C 2 H 5 OH.
- When hydrogen sulfide is passed through cold concentrated sulfuric acid, S and SO 2 are formed, hot concentrated H 2 SO 4 oxidizes sulfur to SO 2. Write the reaction equations. How will the reaction take place between hot concentrated H 2 SO 4 and hydrogen sulfide?
- Why is hydrogen chloride obtained by treating crystalline sodium chloride with concentrated sulfuric acid, while hydrogen bromide and hydrogen iodide are not obtained by this method?
- Make up the equations of the reactions occurring during the interaction of dilute sulfuric acid with a) Zn, b) Al, c) Fe, d) chromium in the absence of oxygen, e) chromium in air.
- Make up the equations of reactions characterizing the redox properties of hydrogen peroxide:
- What reactions occur when the following substances are heated: a) (NH 4) 2 CrO 4; b) NaNO 3; c) CaCO 3; d) Al (NO 3) 3; e) Pb (NO 3) 3; f) AgNO 3; g) Hg (NO 3) 2; i) Cu (NO 3) 2; j) CuO; k) NaClO 4; m) Ca (ClO 4) 2; m) Fe (NO 3) 2; n) PCl 5; p) MnCl 4; c) H 2 C 2 O 4; m) LiNO 3; y) HgO; t) Ca (NO 3) 2; x) Fe (OH) 3; v) CuCl 2; h) KClO 3; w) KClO 2; y) CrO 3?
- When hot solutions of ammonium chloride and potassium nitrate are poured, a reaction occurs, accompanied by gas evolution. Equate this reaction.
- Make up the equations of the reactions occurring when passing through a cold sodium hydroxide solution a) chlorine, b) bromine vapor. The same, but through a hot solution.
- When interacting with a hot concentrated solution of potassium hydroxide, selenium undergoes dismutation to the nearest stable oxidation states (–II and + IV). Make the equation for this OVR.
- Under the same conditions, sulfur undergoes a similar dismutation, but the excess sulfur reacts with sulfite ions to form thiosulfate ions S 2 O 3 2. Make up the equations of the ongoing reactions. ;
- Make up the equations of electrolysis reactions: a) a solution of copper nitrate with a silver anode, b) a solution of lead nitrate with a copper anode.
a) CrCl 2 + FeCl 3; b) CrSO 4 + FeCl 3; c) CrSO 4 + H 2 SO 4 + O 2;
d) CrSO 4 + H 2 SO 4 + MnO 2; e) CrSO 4 + H 2 SO 4 + KMnO 4.
In which of these reactions is hydrogen peroxide an oxidizing agent and in which a reducing agent?
| Experience 1. Oxidizing properties of potassium permanganate in an acidic environment. Add an equal volume of diluted sulfuric acid solution to 3-4 drops of potassium permanganate solution, and then sodium sulfite solution until discoloration. Draw up the reaction equation. Experience 2.Oxidizing properties of potassium permanganate in a neutral environment. Add 5-6 drops of sodium sulfite solution to 3-4 drops of potassium permanganate solution. What substance was precipitated? Test 3. Oxidizing properties of potassium permanganate in an alkaline medium. Add 10 drops of concentrated sodium hydroxide solution and 2 drops of sodium sulfite solution to 3-4 drops of potassium permanganate solution. The solution should turn green. Test 4. Oxidizing properties of potassium dichromate in an acidic environment. Acidify 6 drops of potassium dichromate solution with four drops of dilute sulfuric acid solution and add sodium sulfite solution until the color of the mixture changes. Experience 5. Oxidizing properties of dilute sulfuric acid. Place a zinc granule in one tube and a piece of copper tape in the other. Add 8-10 drops of diluted sulfuric acid solution to both tubes. Compare the events taking place. EXPERIENCE IN A DRAIN CABINET! Experience 6. Oxidizing properties of concentrated sulfuric acid. Similar to experiment 5, but add concentrated sulfuric acid solution. A minute after the beginning of the evolution of gaseous reaction products, introduce strips of filter paper moistened with solutions of potassium permanganate and copper sulfate into the test tubes. Explain what is happening. EXPERIENCE IN A DRAIN CABINET! Experience 7. Oxidizing properties of dilute nitric acid. Similar to experiment 5, but add a dilute solution of nitric acid. Observe the color change of the gaseous reaction products. EXPERIENCE IN A DRAIN CABINET! Test 8. Oxidizing properties of concentrated nitric acid. Place a piece of copper tape in a test tube and add 10 drops of a concentrated solution of nitric acid. Heat gently until the metal is completely dissolved. EXPERIENCE IN A DRAIN CABINET! Test 9. Oxidizing properties of potassium nitrite.To 5-6 drops of potassium nitrite solution add an equal volume of diluted sulfuric acid solution and 5 drops of potassium iodide solution. What substances are formed? Test 10. The reducing properties of potassium nitrite. Add an equal volume of diluted sulfuric acid solution and potassium nitrite solution to 5-6 drops of potassium permanganate solution until the mixture is completely discolored. Experience 11. Thermal decomposition of copper nitrate. Place one microspatula of copper nitrate trihydrate in a test tube, fix it in a rack and gently heat with an open flame. Observe dehydration and subsequent salt decomposition. EXPERIENCE IN A DRAIN CABINET! Test 12. Thermal decomposition of lead nitrate. Carry out analogously to experiment 11, placing lead nitrate in a test tube. EXPERIENCE IN A DRAIN CABINET! What is the difference between the processes taking place during the decomposition of these salts? |
Drawing up equations for redox reactions involving organic substances
IN connection with the introduction of a single form of final certification of high school graduates as the only state examination (Unified State Exam) and the transition of high school to specialized education, it is becoming increasingly important to prepare high school students to perform the most "expensive" in terms of points, tasks of part "C" exam test in chemistry. Although the five tasks of Part C are considered to be different: chemical properties inorganic substances, chains of transformations of organic compounds, computational problems - all of them, to one degree or another, are associated with redox reactions (ORR). If you have mastered the basic knowledge of the theory of OVR, then you can correctly complete the first and second tasks in full, and the third - in part. In our opinion, this is a significant part of the success of Part C. Experience shows that if, while studying inorganic chemistry, students cope well enough with the tasks of writing the OVR equations, then similar tasks for organic chemistry cause them great difficulties. Therefore, throughout the study of the entire course of organic chemistry in specialized classes, we try to form the skills of drawing up OVR equations in high school students.
When studying comparative characteristics of inorganic and organic compounds, we introduce students to the use of the oxidation state (s.o.) (in organic chemistry, primarily carbon) and methods of its determination:
1) calculation of the average s.o. carbon in a molecule of organic matter;
2) definition of s.o. every carbon atom.
We clarify in which cases it is better to use this or that method.
The article was published with the support of the company "GEO-Engineering", which represents on the market products under the brand "ProfKresla". The scope of the company is the production, sale and installation of armchairs and chairs for various rooms. High professionalism of employees and our own production facilities allow us to quickly and efficiently implement projects of any complexity. All products under the brand "ProfKresla", be it theater chairs, seating for waiting rooms or chairs for educational institutions, are distinguished by a modern and ergonomic design, as well as high wear resistance, strength and comfort. From the huge range of products presented in the catalog on the profkresla.ru website, you can always choose the models that best suit the corporate style adopted in your company. If you still have difficulties with the choice, then the company's specialists are always ready to give advice, help determine the model, then prepare a project, make all the necessary measurements and installation on the spot.
P When studying the topic "Alkanes" we show that the processes of oxidation, combustion, halogenation, nitration, dehydrogenation, decomposition are redox processes. When writing the equations for the reactions of combustion and decomposition of organic substances, it is better to use the average value of the s.r. carbon. For instance:
Paying attention to the first half electronic balance: at the carbon atom in the fractional value of s.o. the denominator is 4, so we calculate the electron transfer using this coefficient.
In other cases, when studying the topic "Alkanes", we determine the values \u200b\u200bof s.o. of each carbon atom in the compound, while drawing the students' attention to the sequence of substitution of hydrogen atoms in primary, secondary, tertiary carbon atoms:


Thus, we bring the students to the conclusion that at the beginning the process of substitution takes place at tertiary atoms, then at secondary ones, and, last of all, at primary carbon atoms.
P When studying the topic "Alkenes", we consider the oxidation processes depending on the structure of the alkene and the reaction medium.
During the oxidation of alkenes with a concentrated solution of potassium permanganate KMnO 4 in an acidic medium (severe oxidation), rupture of - and - bonds occurs with the formation of carboxylic acids, ketones and carbon monoxide (IV). This reaction is used to determine the position of the double bond.
If the double bond is at the end of the molecule (for example, in butene-1), then one of the oxidation products is formic acid, which is easily oxidized to carbon dioxide and water:

We emphasize that if in an alkene molecule a carbon atom at a double bond contains two carbon substituents (for example, in a 2-methylbutene-2 \u200b\u200bmolecule), then a ketone is formed during its oxidation, since the transformation of such an atom into a carboxyl group atom is impossible without breaking C – C-bond, relatively stable under these conditions:

We clarify that if the alkene molecule is symmetric and the double bond is contained in the middle of the molecule, then only one acid is formed during oxidation:

We report that a feature of the oxidation of alkenes, in which the carbon atoms at the double bond contain two carbon radicals, is the formation of two ketones:


Considering the oxidation of alkenes in neutral or slightly alkaline media, we focus the attention of senior students on the fact that under such conditions oxidation is accompanied by the formation of diols (dihydric alcohols), and hydroxyl groups are attached to those carbon atoms between which there was a double bond:

IN Similarly, we consider the oxidation of acetylene and its homologues, depending on the environment in which the process takes place. So, we clarify that in an acidic environment, the oxidation process is accompanied by the formation of carboxylic acids:

The reaction is used to determine the structure of alkynes by oxidation products:

In neutral and slightly alkaline media, the oxidation of acetylene is accompanied by the formation of the corresponding oxalates (salts of oxalic acid), and the oxidation of homologues is accompanied by the breaking of the triple bond and the formation of salts of carboxylic acids:

IN All the rules are worked out with students on specific examples, which leads to a better assimilation of theoretical material. Therefore, when studying the oxidation of arenes in various environments, students can independently make assumptions that the formation of acids should be expected in an acidic environment, and salts in an alkaline environment. The teacher will only have to clarify what reaction products are formed depending on the structure of the corresponding arena.
We show by examples that homologues of benzene with one side chain (regardless of its length) are oxidized by a strong oxidizing agent to benzoic acid at the -carbon atom. On heating, benzene homologues are oxidized by potassium permanganate in a neutral medium with the formation of potassium salts of aromatic acids.
5C 6 H 5 –CH 3 + 6KMnO 4 + 9H 2 SO 4 \u003d 5C 6 H 5 COOH + 6MnSO 4 + 3K 2 SO 4 + 14H 2 O,
5C 6 H 5 –C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 \u003d 5C 6 H 5 COOH + 5CO 2 + 12MnSO 4 + 6K 2 SO 4 + 28H 2 O,
C 6 H 5 –CH 3 + 2KMnO 4 \u003d C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O.
We emphasize that if there are several side chains in the arene molecule, then in an acidic medium each of them is oxidized at the a-carbon atom to a carboxyl group, as a result of which polybasic aromatic acids are formed:

P The acquired skills of drawing up the OVR equations for hydrocarbons make it possible to use them when studying the section “Oxygen-containing compounds”.
So, when studying the topic "Alcohols", students independently compose equations for the oxidation of alcohols using the following rules:
1) primary alcohols are oxidized to aldehydes
3CH 3 –CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 –CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O;
2) secondary alcohols are oxidized to ketones

3) the oxidation reaction is not typical for tertiary alcohols.
In order to prepare for the Unified State Exam, it is advisable for the teacher to give additional information to the indicated properties, which will undoubtedly be useful for students.
When methanol is oxidized with an acidified solution of potassium permanganate or potassium dichromate, CO 2 is formed, primary alcohols during oxidation, depending on the reaction conditions, can form not only aldehydes, but also acids. For example, the oxidation of ethanol with potassium dichromate in the cold ends with the formation of acetic acid, and when heated - acetaldehyde:
3CH 3 –CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 \u003d 3CH 3 –COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O,
3CH 3 –CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 3CH 3 –CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O.
Let us remind students again about the influence of the environment on the products of alcohol oxidation reactions, namely: a hot neutral solution of KMnO 4 oxidizes methanol to potassium carbonate, and other alcohols - to salts of the corresponding carboxylic acids:

When studying the topic "Aldehydes and ketones", we focus the attention of students on the fact that aldehydes are easier than alcohols to oxidize to the corresponding carboxylic acids not only under the action of strong oxidants (air oxygen, acidified solutions of KMnO 4 and K 2 Cr 2 O 7), but and under the influence of weak (ammonia solution of silver oxide or copper (II) hydroxide):
5CH 3 –CHO + 2KMnO 4 + 3H 2 SO 4 \u003d 5CH 3 –COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O,
3CH 3 –CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 –COOH + Cr 2 (SO 4) 3 + K 2 SO 4 + 4H 2 O,
CH 3 –CHO + 2OH CH 3 –COONH 4 + 2Ag + 3NH 3 + H 2 O.
We pay special attention to the oxidation of methanal with an ammonia solution of silver oxide, because in this case, ammonium carbonate is formed, and not formic acid:
HCHO + 4OH \u003d (NH 4) 2 CO 3 + 4Ag + 6NH 3 + 2H 2 O.
As our long-term experience shows, the proposed methodology for teaching high school students to compose OVR equations with the participation of organic substances increases their final USE result in chemistry by several points.
Redox reactions in organic chemistry are of greatest interest, because the transition from one oxidation state to another strongly depends on the correct choice of the reagent and the reaction conditions. OVR is studied in a compulsory chemistry course not fully enough, but in control and measuring materials of the exam are found not only in tasks C1 and C2, but also tasks SZ, representing a chain of transformations of organic substances.
Download:
Preview:
To use the preview of presentations, create yourself a Google account (account) and log into it: https://accounts.google.com
Slide captions:
OXIDATION-REDUCTION REACTIONS IN ORGANIC CHEMISTRY
“It is easy to think, it is difficult to act, and turning thought into action is the most difficult thing in the world” I. Goethe Redox reactions in organic chemistry are of the greatest interest, because the selectivity of the transition from one oxidation state to another strongly depends on the correct choice of reagent and reaction conditions. But OVR is not studied fully in the compulsory chemistry course. Students should pay special attention to the redox processes that occur with the participation of organic substances. This is due to the fact that redox reactions in the control and measuring materials of the exam are found not only in tasks C1 and C2, but also in tasks SZ, which represent a chain of transformations of organic substances. In school textbooks, the oxidizing agent is often written above the arrow as [O]. The requirement for completing such tasks on the USE is the mandatory designation of all starting materials and reaction products with the placement of the necessary coefficients. Redox reactions are traditionally important, and at the same time, studying in grade 10, in the course "Organic Chemistry", causes certain difficulties for students.
C3. The tasks of this block test the knowledge of organic chemistry. In the chains of transformations of organic substances, the overwhelming majority of tasks contain OVR. The expert has the right to assign a point only if the equation is written down, and not the reaction scheme, i.e. the coefficients are correctly placed. In reactions involving inorganic oxidants (potassium permanganate, chromium (VI) compounds, hydrogen peroxide, etc.), this can be difficult to do, without electronic balance.
Determination of the oxidation state of atoms in molecules of organic compounds RULE: CO (atom) \u003d the number of bonds with more EO atoms minus the number of bonds with less EO atoms.
Change in the oxidation state of carbon atoms in molecules of organic compounds. Class of organic compounds The oxidation state of the carbon atom -4 / -3 -2 -1 0 +1 +2 +3 +4 Alkanes CH 4 CH 3 -CH 3 CH 3 -CH 2 -CH 3 CH 3 | C H 3 -C H-CH 3 CH 3 | C H 3 -C -CH 3 | CH 3 - - - - Alkenes - CH 2 \u003d CH 2 CH 3 -CH \u003d CH 2 - - - - Alkines - - CH \u003d CH CH 3 -C \u003d CH - - - - Alcohols _ _ H 3 C-CH 2 - OH H 3 CC H-CH 3 | OH CH 3 | H 3 C - C - CH 3 | OH - - - Halogenalkanes - - H 3 C-CH 2 - CI H 3 C - C H - CH 3 | CI CH 3 | H 3 C - C - CH 3 | CI - - - Aldehydes and ketones - - - - H 3 C-CH \u003d O H 3 C-C OCH 3 - - Carboxylic acids - - - - - - H 3 C-C OOH - Complete oxidation products - - - - - - - CO 2
The tendency of organic compounds to oxidize is associated with the presence of: multiple bonds (alkenes, alkynes, alkadienes are easily oxidized); functional groups that can be easily oxidized (–OH, - CHO, - NH 2); activated alkyl groups located adjacent to multiple bonds or a benzene ring (for example, propene can be oxidized to unsaturated acrolein aldehyde, oxidation of toluene to benzoic acid with potassium permanganate in an acidic medium); the presence of hydrogen atoms at the carbon atom containing the functional group.
1. SOFT OXIDATION OF ORGANIC COMPOUNDS For mild oxidation of organic compounds (alcohols, aldehydes, unsaturated compounds), chromium (VI) compounds are used - chromium (VI) oxide, CrO 3, potassium dichromate К 2 С r 2 O 7, etc. As a rule, oxidation is carried out in an acidic environment, the reduction products are chromium (III) salts, for example: 3CH 3 –CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 –COOH + 4K 2 SO 4 + Cr 2 (SO 4) 3 + 4H 2 O t 3CH 3 –CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 → 3CH 3 –COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O Upon oxidation of alcohols with dichromate potassium in the cold, oxidation can be stopped at the stage of aldehyde formation, while heating carboxylic acids are formed: 3CH 3 –CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 → 3CH 3 –C HO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O
ALK EH + KMnO4 -1 KOH N 2SO4 Diol Carboxylic acid salt + carbonate Carbonic acid + CO 2 ALK EH + KMnO4 -2 KOH H 2SO4 2 carboxylic acid salts 2 carbonic acid Diol 2 much stronger the oxidizing agent is potassium permanganate NEUTR. NEUTR.
C 2 H 2 + 2KMnO 4 + 3H 2 SO 4 \u003d 2CO 2 + 2MnSO 4 + 4H 2 O + K 2 SO 4 ALK IN + KMnO4 -1 KOH Н 2SO4 Carboxylic acid salt + carbonate Carbonic acid + СО 2 ALK IN + KMnO4 -2 KOH H 2SO4 2 salts of carb. to-you 2 carbon-to-you 5CH 3 C \u003d CH + 8KMnO 4 + 12H 2 SO 4 \u003d 5CH 3 COOH + 5CO 2 + 8MnSO 4 + 4K 2 SO 4 + 12H 2 O
5C 6 H 5 -CH 3 +6 KMnO 4 + H 2 SO 4 5C 6 H 5 COOH + 6MnSO 4 + K 2 SO 4 + 14H 2 OC 6 H 5 CH 3 + 2KMnO 4 C 6 H 5 COOK + 2MnO 2 + KOH + H 2 OC 6 H 5 CH 2 CH 3 + 4KMnO 4 C 6 H 5 COOK + K 2 CO 3 + 2H 2 O + 4MnO 2 + KOH Benzene homologues + KMnO4 KOH H 2SO4 benzoic acid NEUTR. Benzoate
Redox properties of oxygen-containing compounds Oxidizing agents of alcohols are most often copper (II) oxide or potassium permanganate, and oxidizing agents of aldehydes and ketones - copper (II) hydroxide, ammonia solution of silver oxide and other oxidizing agents
OL + KMnO4 -1 KOH H 2SO4 ALDEHYDE OL + KMnO4 -2 KOH N 2SO4 ketone OL + K MnO4 (ex) -1 KOH N 2SO4 NEUTR Carboxylic acid salt Carboxylic acid salt Carboxylic acid
Aldehyde + KMnO4 KOH H 2SO4 Carboxylic acid + carboxylic acid salt Carboxylic acid salt carboxylic acid NEUTR. 3CH 3 CHO + 2KMnO 4 \u003d CH 3 COOH + 2CH 3 COOK + 2MnO 2 + H 2 O
Aldehydes are quite strong reducing agents, and therefore are easily oxidized by various oxidants CH 3 CHO + 2OH CH 3 COONH 4 + 2Ag + H 2 O + 3NH 3
Algorithm for the selection of coefficients Since task C3 does not require writing electronic balance equations when drawing up the OVR equations, it is convenient to select the coefficients using the subscript balance method - a simplified electronic balance method. 1 . An OVR scheme is drawn up. For example, for the oxidation of toluene to benzoic acid with an acidified solution of potassium permanganate, the reaction scheme is as follows: C 6 H 5 -CH 3 + KMnO 4 + H 2 SO 4 C 6 H 5 -C OO H + K 2 SO 4 + MnSO 4 + H 2 O 2. Indicated with. atoms. S.o. the carbon atom is determined according to the above method. C 6 H 5 -C -3 H 3 + KMn +7 O 4 + H 2 SO 4 C 6 H 5 -C +3 OO H + K 2 SO 4 + Mn +2 SO 4 + H 2 O 3. Number electrons donated by a carbon atom (6) is written as a coefficient in front of the formula of the oxidizing agent (potassium permanganate): C 6 H 5 -C -3 H 3 + 6 KMn +7 O 4 + H 2 SO 4 C 6 H 5 -C + 3 OO H + K 2 SO 4 + Mn + 2 SO 4 + H 2 O 4. The number of electrons taken by the manganese atom (5) is written as a coefficient before the formula of the reducing agent (toluene): 5 C 6 H 5 -C -3 H 3 + 6 KMn +7 O 4 + H 2 SO 4 C 6 H 5 -C +3 OO H + K 2 SO 4 + Mn +2 SO 4 + H 2 O 5. The most important coefficients are in place. Further selection is not difficult: 5 C 6 H 5 -CH 3 + 6 KMnO 4 + 9 H 2 SO 4 5 C 6 H 5 -C OO H + 3 K 2 SO 4 + 6 MnSO 4 + 14 H 2 O
An example of a test task (C3) 1. Write the reaction equations that can be used to carry out the following transformations: Hg 2+, H + KMnO 4, H + C l 2 (equimolar), h C 2 H 2 X 1 CH 3 COOH X 2 CH 4 X 3 1. Kucherov reaction. Hg 2+, H + CH CH + H 2 O CH 3 CHO 2. Aldehydes are easily oxidized to carboxylic acids, including such a strong oxidizing agent as potassium permanganate in an acidic environment. CH 3 CHO + KMnO 4 + H 2 SO 4 CH 3 COOH + K 2 SO 4 + MnSO 4 + H 2 O CH 3 C +1 H O + KMn +7 O 4 + H 2 SO 4 CH 3 -C +3 OO Н + K 2 SO 4 + Mn +2 SO 4 + H 2 O 5 CH 3 CHO + 2 KMnO 4 + 3 H 2 SO 4 5 CH 3 COOH + K 2 SO 4 + 2 MnSO 4 + 3 H 2 O 3. To perform the next link in the chain, it is necessary to evaluate the substance X 2 from two positions: firstly, it is formed in one stage from acetic acid, and secondly, methane can be obtained from it. This substance is acetate alkali metal... The equations of the third and fourth reactions are written. CH 3 COOH + NaOH CH 3 COONa + H 2 O fusion 4. CH 3 COONa + NaOH CH 4 + Na 2 CO 3 5. The conditions for the next reaction (light) unambiguously indicate its radical nature. Taking into account the indicated ratio of reagents (equimolar), the equation of the last reaction is written: h CH 4 + Cl 2 CH 3 Cl + HCl
Simulator sites: http://reshuege.ru/ (I will solve the Unified State Exam) http://4ege.ru/himiya/4181-demoversiya-ege-po-himii-2014.html (Unified State Exam portal) http: //www.alleng. ru / edu / chem3.htm ( Educational resources Internet - Chemistry) http://ege.yandex.ru/ (online tests)