Classification organic compounds
To classify organic compounds by types and construct their names in the molecule of an organic compound, it is customary to distinguish carbon skeletonand functional groups.
The carbon skeleton is a sequence of chemically bonded carbon atoms. Functional groups are atoms of elements other than hydrogen or groups of atoms bonded to carbon atoms.
Depending on the structure of the carbon skeleton, organic compounds are divided into acyclicand cyclical.
Acyclic compounds - connections with open ( open)carbon chain; they can be saturated (alkanes and their derivatives) and unsaturated (alkenes, alkadienes, alkynes and their derivatives).
Acyclic skeletons are unbranched(for example, in n-pentane) and ramified(for example, in 2,3-dimethylbutane):
Cyclic connections - closed circuit connections. Depending on the nature of the atoms that make up the cycle, there are carbocyclicand heterocyclicconnections.
Carbocyclic compounds contain only carbon atoms in the cycle and are divided into two groups that differ significantly in chemical properties: aliphatic cyclic(abbreviated as alicyclic) and aromaticconnections.
The simplest representative of saturated alicyclic hydrocarbons (cycloalkanes) is cyclopropane containing a three-membered ring. The number of carbon atoms in the cycles can be different. Large cycles (macrocycles) are known, consisting of 30 or more carbon atoms.
The progenitor of aromatic hydrocarbons (arenes) is benzene. Naphthalene and phenanthrene are polycyclic arenas; they contain benzene rings that have common bonds (another name for these compounds is condensed arenes.
Heterocyclic connectionscontain in the cycle, in addition to carbon atoms, one or more atoms of other elements - heteroatoms (from the Greek heteros - other, different) - oxygen, nitrogen, sulfur, etc.
In the carbon skeletons themselves, it is useful to classify individual carbon atoms by the number of carbon atoms chemically bonded to them. If a given carbon atom is bonded to onecarbon atom, then it is called primary,from two– secondary, three– tertiaryand four– quaternary.
Since carbon atoms can form among themselves not only single bonds, but also multiple (double and triple) bonds, compounds containing only single carbon-carbon bonds are called saturated,compounds with multiple carbon-carbon bonds are called unsaturated.Compounds in which carbon atoms are bonded only to hydrogen atoms are called hydrocarbons.
Hydrocarbons are recognized in organic chemistry ancestral structures.Various compounds are considered as derivatives of hydrocarbons obtained by introducing functional groups into them.
Functional groups - atoms or their groups, which largely determine the chemical and physical properties organic compounds . Compounds that contain several functional groups are called polyfunctional.
Compounds that have the same functional groups, but differ in the number of carbon atoms, have very similar physical and chemical properties. Homologues– these are compounds belonging to the same class, but differing from each other in composition by an integer number of groupsCH 2. The set of all homologues forms homologous series.
Currently recognized systematic nomenclature IUPAC (IURAC - International Union of Pure and Applied Chemistry). Among the systematic nomenclatures recommended by IUPAC, the most common is substitutional nomenclature.
According to the IUPAC rulesthe name of an organic compound is constructed from the name of the main chain forming the root of the word and the names of functions used as prefixes or suffixes.
For correct construction names, it is necessary to select the main chain and the numbering of carbon atoms in it.
In substitutional nomenclature, the name of a compound is a compound word, the root of which includes the name of the parent structure. The names of substituents are indicated by prefixes (prefixes) and suffixes.
A substituent is any atom or group of atoms that replaces a hydrogen atom in the parent structure.
Functional groupIs an atom or group of atoms of a non-hydrocarbon character that determines the belonging of a compound to a certain class.
Characteristic groupIs a functional group associated with the parent structure. To construct a name, first of all, the type of characteristic group is determined (if it is present) .When there are several characteristic groups in the compound, then the senior characteristic group.The order of precedence is conditionally established for characteristic groups. In the table, these groups are listed in descending order of seniority. Then the parental structure is determined, which must include the senior characteristic group.
Some characteristic groups, namely halogens, nitro and alkoxy groups, are reflected in the general name only in the form of prefixes, for example, bromomethane, ethoxyethane, nitrobenzene.
Numbering of carbon atoms in the main chainstart from the end of the chain closer to which is located senior group... If there are several such possibilities, then the numbering is carried out in such a way that either the multiple bond or another substituent present in the molecule is given the lowest number.
IN carbocycliccompounds, the numbering begins from the carbon atom at which the most significant characteristic group is located. If it is not possible to choose an unambiguous numbering, then the cycle is numbered so that the substituents have the lowest numbers.
In the group of cyclic hydrocarbons, aromatic hydrocarbons,which are characterized by the presence of a benzene ring in the molecule. Some well-known representatives of aromatic hydrocarbons and their derivatives have trivial names, the use of which is permitted by the IUPAC rules: benzene, toluene, phenol, benzoic acid.
It should be noted that the C 6 H 5 - radical formed from benzene is called phenyl,not benzyl. Benzylcall the radical C 6 H 5 CH 2 - , formed from toluene.
Formation of the name of the organic compound.The basis of the name of the compound is the root of the word denoting a saturated hydrocarbon with the same number of atoms as the main chain: for example, one carbon atom - met-,two - et-,three - prop-,four - but-,five - pent-,six - hex-etc.This is followed by a suffix characterizing the degree of saturation, -an,if there are no multiple bonds in the molecule, -enin the presence of double bonds and -infor triple bonds, eg pentane, pentene, pentine. If there are several multiple bonds in a molecule, then the number of such bonds is indicated in the suffix, for example: -diene, -triene,and after the suffix, the position of the multiple bond must be indicated in Arabic numerals (for example, butene-1, butene-2, butadiene-1,3):
Further, the name of the most senior characteristic group in the molecule is entered into the suffix, indicating its position with a number. Other substituents are designated with prefixes. Moreover, they are listed not in order of seniority, but alphabetically. The position of the substituent is indicated by a number before the prefix, for example: 3-methyl; 2-chlorine, etc. If there are several identical substituents in a molecule, then their number is indicated before the name of the corresponding group (for example, dimethyl-, trichloro-, etc.). All numbers in the names of molecules are separated from words by a hyphen, and from each other by commas. Hydrocarbon radicals have their own names.
As an example, let's call the following connection:
1) The choice of the chain is unambiguous, therefore, the root of the word is pent;followed by a suffix -en,indicating the presence of a multiple connection;
2) the order of numbering provides the oldest group (-OH) with the lowest number;
3) the full name of the compound ends with a suffix denoting an older group (in this case, the suffix -olindicates the presence of a hydroxyl group); the position of the double bond and hydroxyl group is indicated by numbers.
Therefore, the above compound is called penten-4-ol-2.
Rational and functional nomenclature used for halogen derivatives, alcohols, ethers and especially amines:
In addition, the historically established trivial names of organic compounds (for example: acetone, acetic acid, formaldehyde, etc.) are still widely used. The most important trivial names are introduced in the text when considering the corresponding classes of compounds.
Classification of organic substances
Depending on the type of structure of the carbon chain, organic substances are divided into:
- acyclic and cyclic.
- limiting (saturated) and unsaturated (unsaturated).
- carbocyclic and heterocyclic.
- alicyclic and aromatic.
Acyclic compounds are organic compounds in the molecules of which there are no cycles and all carbon atoms are connected to each other in straight or branched open chains.
In turn, among the acyclic compounds, limiting (or saturated) ones are distinguished, which contain in the carbon skeleton only single carbon-carbon (C-C) bonds and unsaturated (or unsaturated), containing multiple - double (C \u003d C) or triple (C≡ C) communication.
Cyclic connections - chemical compounds, in which there are three or more bonded atoms to form a ring.
Depending on which atoms form the rings, carbocyclic compounds and heterocyclic compounds are distinguished.
Carbocyclic compounds (or isocyclic) contain only carbon atoms in their rings. These compounds, in turn, are divided into alicyclic compounds (aliphatic cyclic) and aromatic compounds.
Heterocyclic compounds contain one or more heteroatoms in the hydrocarbon cycle, most often oxygen, nitrogen or sulfur atoms.
The simplest class of organic substances are hydrocarbons - compounds that are formed exclusively by carbon and hydrogen atoms, i.e. formally do not have functional groups.
Since hydrocarbons do not have functional groups, only classification according to the type of carbon skeleton is possible. Hydrocarbons, depending on the type of their carbon skeleton, are divided into subclasses:
1) Saturated acyclic hydrocarbons are called alkanes. The general molecular formula of alkanes is written as C n H 2n + 2, where n is the number of carbon atoms in a hydrocarbon molecule. These compounds do not have interclass isomers.
2) Acyclic unsaturated hydrocarbons are divided into:
a) alkenes - they contain only one multiple, namely one double C \u003d C bond, the general formula for alkenes is C n H 2n,
b) alkynes - in the molecules of alkynes there is also only one multiple, namely the triple C≡C bond. General molecular formula of alkynes C n H 2n-2
c) alkadienes - in the molecules of alkadienes there are two double C \u003d C bonds. General molecular formula of alkadienes C n H 2n-2
3) Cyclic saturated hydrocarbons are called cycloalkanes and have the general molecular formula C n H 2n.
The rest of organic substances in organic chemistry are considered as derivatives of hydrocarbons, formed when the so-called functional groups are introduced into the molecules of hydrocarbons, which contain other chemical elements.
Thus, the formula for compounds with one functional group can be written as R-X, where R is a hydrocarbon radical and X is a functional group. A hydrocarbon radical is a fragment of a molecule of any hydrocarbon without one or more hydrogen atoms.
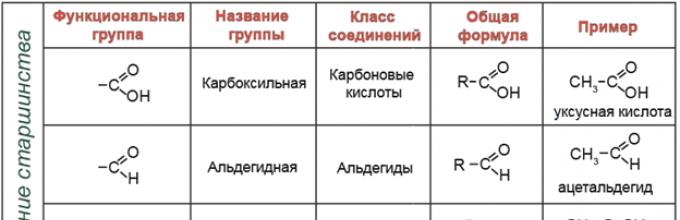
According to the presence of certain functional groups, compounds are divided into classes. The main functional groups and classes of compounds, which include them, are presented in the table:

Thus, various combinations of types of carbon skeletons with different functional groups give a wide variety of variants of organic compounds.
Halogenated hydrocarbons
Halogenated hydrocarbons are compounds obtained by replacing one or more hydrogen atoms in the molecule of any starting hydrocarbon with one or more atoms of any halogen, respectively.
Let some hydrocarbon have the formula C n H m, then when replacing in its molecule X hydrogen atoms per X halogen atoms, the formula of the halogen derivative will have the form C n H m- X Hal X ... Thus, monochloro derivatives of alkanes have the formula C n H 2n + 1 Cl, dichloro derivatives C n H 2n Cl 2 etc.
Alcohols and phenols
Alcohols are derivatives of hydrocarbons in which one or more hydrogen atoms are replaced by the hydroxyl group -OH. Alcohols with one hydroxyl group are called monoatomic, with two - diatomic, with three triatomic etc. For instance:
Alcohols with two or more hydroxyl groups are also called polyhydric alcohols.General formula of saturated monohydric alcohols C n H 2n + 1 OH or C n H 2n + 2 O. General formula of saturated polyhydric alcohols C n H 2n + 2 O x, where x is the atomicity of alcohol.
Alcohols can also be aromatic. For instance:
benzyl alcoholThe general formula of such monohydric aromatic alcohols is C n H 2n-6 O.
However, it should be clearly understood that derivatives of aromatic hydrocarbons in which hydroxyl groups are replaced by one or more hydrogen atoms in the aromatic nucleus do not apply to alcohols. They belong to the class phenols ... For example, this given compound is an alcohol:
And this is phenol:
The reason why phenols are not classified as alcohols lies in their specific chemical propertiesah, very different from alcohols. It is easy to see that monohydric phenols are isomeric to monohydric aromatic alcohols, i.e. also have the general molecular formula C n H 2n-6 O.
Amines
Aminami called derivatives of ammonia, in which one, two or all three hydrogen atoms are replaced by a hydrocarbon radical.
Amines in which only one hydrogen atom is replaced by a hydrocarbon radical, i.e. having the general formula R-NH 2 are called primary amines.
Amines in which two hydrogen atoms are replaced by hydrocarbon radicals are called secondary amines... The secondary amine formula can be written as R-NH-R '. In this case, the radicals R and R 'can be either the same or different. For instance:
If amines contain no hydrogen atoms at the nitrogen atom, i.e. all three hydrogen atoms of the ammonia molecule are replaced by a hydrocarbon radical, then such amines are called tertiary amines... In general, the tertiary amine formula can be written as:
In this case, the radicals R, R ', R' 'can be either completely the same or all three different.
General molecular formula of primary, secondary and tertiary limit amines has the form C n H 2 n +3 N.
Aromatic amines with only one unsaturated substituent have the general formula C n H 2 n -5 N
Aldehydes and ketones
Aldehydes are called derivatives of hydrocarbons, in which at the primary carbon atom two hydrogen atoms are replaced by one oxygen atom, i.e. derivatives of hydrocarbons in the structure of which there is an aldehyde group –CH \u003d O. The general formula for aldehydes can be written as R-CH \u003d O. For instance:
Ketones derivatives of hydrocarbons are called, in which at the secondary carbon atom two hydrogen atoms are replaced by an oxygen atom, i.e. compounds in the structure of which there is a carbonyl group –C (O) -.
The general formula for ketones can be written as R-C (O) -R '. In this case, the radicals R, R 'can be either the same or different.
For instance:
| propane it | butane it |
As you can see, aldehydes and ketones are very similar in structure, but they are still distinguished as classes, since they have significant differences in chemical properties.
The general molecular formula of saturated ketones and aldehydes is the same and has the form C n H 2 n O
Carboxylic acids
Carboxylic acids are called derivatives of hydrocarbons, which have a carboxyl group -COOH.
If an acid has two carboxyl groups, this acid is called dicarboxylic acid.
Saturated monocarboxylic acids (with one group -COOH) have a general molecular formula of the form C n H 2 n O 2
Aromatic monocarboxylic acids have the general formula C n H 2 n -8 O 2
Ethers
Ethers -organic compounds in which two hydrocarbon radicals are indirectly linked through an oxygen atom, i.e. have the formula of the form R-O-R '. In this case, the radicals R and R 'can be either the same or different.
For instance:
The general formula for saturated ethers is the same as for saturated monohydric alcohols, i.e. C n H 2 n +1 OH or C n H 2 n +2 O.
Esters
Esters are a class of compounds based on organic carboxylic acids in which the hydrogen atom in the hydroxyl group is replaced by a hydrocarbon radical R. The formula of esters in general form can be written as:
For instance:
Nitro compounds
Nitro compounds - derivatives of hydrocarbons, in which one or more hydrogen atoms are replaced by the nitro group –NO 2.
Limit nitro compounds with one nitro group have the general molecular formula C n H 2 n +1 NO 2
Amino acids
Compounds having in their structure simultaneously two functional groups - amino NH 2 and carboxyl - COOH. For instance,
NH 2 -CH 2 -COOH
Limit amino acids with one carboxyl and one amino group are isomeric to the corresponding limiting nitro compounds, i.e. as they have the general molecular formula C n H 2 n +1 NO 2
IN tasks of the exam for the classification of organic substances, it is important to be able to write down the general molecular formulas of homologous series of different types of compounds, knowing the structural features of the carbon skeleton and the presence of certain functional groups. In order to learn how to determine the general molecular formulas of organic compounds of different classes, the material on this topic will be useful.
Nomenclature of organic compounds
Features of the structure and chemical properties of compounds are reflected in the nomenclature. The main types of items are considered systematic and trivial.
The systematic nomenclature actually prescribes algorithms, according to which a particular name is compiled in strict accordance with the structural features of the molecule of organic matter or, roughly speaking, its structural formula.
Consider the rules for compiling the names of organic compounds according to the systematic nomenclature.
When compiling the names of organic substances according to the systematic nomenclature, the most important thing is to correctly determine the number of carbon atoms in the longest carbon chain or to count the number of carbon atoms in a cycle.
Depending on the number of carbon atoms in the main carbon chain, the compounds will have a different root in their name:
|
The number of C atoms in the main carbon chain |
Name root |
|
prop- |
|
|
pent- |
|
|
hex- |
|
|
hept- |
|
|
dec (c) - |
The second important component taken into account when composing names is the presence / absence of multiple bonds or functional groups, which are listed in the table above.
Let's try to give a name to a substance that has a structural formula:
1. The main (and only) carbon chain of this molecule contains 4 carbon atoms, so the name will contain the root but-;
2. There are no multiple bonds in the carbon skeleton, therefore, the suffix that needs to be used after the root of the word will be -an, as in the corresponding saturated acyclic hydrocarbons (alkanes);
3. The presence of a functional group –OH, provided that there are no more senior functional groups, adds after the root and the suffix from item 2. one more suffix - "ol";
4. In molecules containing multiple bonds or functional groups, the numbering of the carbon atoms of the main chain starts from the side of the molecule to which they are closer.
Let's look at another example:
The presence of four carbon atoms in the main carbon chain tells us that the root of the name is “but-”, and the absence of multiple bonds indicates the suffix “-an”, which will follow immediately after the root. The older group in this compound is carboxyl, which determines the belonging of this substance to the class of carboxylic acids. Therefore, the ending of the name will be "-oic acid". There is an amino group at the second carbon atom NH 2 -, therefore, this substance belongs to amino acids. Also at the third carbon atom, we see the hydrocarbon radical methyl ( CH 3 -). Therefore, according to the systematic nomenclature, this compound is called 2-amino-3-methylbutanoic acid.
The trivial nomenclature, in contrast to the systematic, as a rule, has no connection with the structure of a substance, but is mainly due to its origin, as well as chemical or physical properties.
| Formula | Systematic nomenclature name | Trivial name |
| Hydrocarbons | ||
| CH 4 | methane | marsh gas |
| CH 2 \u003d CH 2 | ethen | ethylene |
| CH 2 \u003d CH-CH 3 | propene | propylene |
| CH≡CH | ethine | acetylene |
| CH 2 \u003d CH-CH \u003d CH 2 | butadiene-1,3 | divinyl |
| 2-methylbutadiene-1,3 | isoprene | |
| methylbenzene | toluene | |
| 1,2-dimethylbenzene | ortho-xylene (about-xylene) |
|
| 1,3-dimethylbenzene | meta-xylene (m-xylene) |
|
| 1,4-dimethylbenzene | couple-xylene (p-xylene) |
|
| vinylbenzene | styrene | |
| Alcohols | ||
| CH 3 OH | methanol | methyl alcohol, wood alcohol |
| CH 3 CH 2 OH | ethanol | ethanol |
| CH 2 \u003d CH-CH 2 -OH | propene-2-ol-1 | allyl alcohol |
| ethanediol-1,2 | ethylene glycol | |
| propanetriol-1,2,3 | glycerol | |
| phenol (hydroxybenzene) |
carbolic acid | |
| 1-hydroxy-2-methylbenzene | ortho-cresol (about-cresol) |
|
| 1-hydroxy-3-methylbenzene | meta-cresol (m-cresol) |
|
| 1-hydroxy-4-methylbenzene | couple-cresol (P-cresol) |
|
| phenylmethanol | benzyl alcohol | |
| Aldehydes and ketones | ||
| methanal | formaldehyde | |
| ethanal | acetaldehyde, acetaldehyde | |
| propenal | acrylic aldehyde, acrolein | |
| benzaldehyde | benzoic aldehyde | |
| propanone | acetone | |
| Carboxylic acids | ||
| (HCOOH) | methanoic acid | formic acid (salts and esters - formates) |
| (CH 3 COOH) | ethanic acid | acetic acid (salts and esters - acetates) |
| (CH 3 CH 2 COOH) | propanoic acid | propionic acid (salts and esters - propionates) |
| C 15 H 31 COOH | hexadecanoic acid | palmitic acid (salts and esters - palmitates) |
| C 17 H 35 COOH | octadecanoic acid | stearic acid (salts and esters - stearates) |
| propenoic acid | acrylic acid (salts and esters - acrylates) |
|
| HOOC-COOH | ethanedioic acid | oxalic acid (salts and esters - oxalates) |
| 1,4-benzenedicarboxylic acid | terephthalic acid | |
| Esters | ||
| HCOOCH 3 | methylmetanoate | methyl formate, muric acid methyl ester |
| CH 3 COOCH 3 | methylethanoate | methyl acetate, acetic acid methyl ester |
| CH 3 COOC 2 H 5 | ethyl ethanoate | ethyl acetate, ethyl acetate |
| CH 2 \u003d CH-COOCH 3 | methylpropenoate | methyl acrylate, acrylic acid methyl ester |
| Nitrogen compounds | ||
| aminobenzene, phenylamine |
aniline | |
| NH 2 -CH 2 -COOH | aminoethanoic acid | glycine, aminoacetic acid |
| 2-aminopropionic acid | alanine | |
Several systems are used to name organic compounds, but none of them are suitable for all compounds. Many trivial names have survived, which were either used back in initial period organic chemistry and reflect the source of production or characteristic qualities, or are newer non-systematic names that are used for convenience reasons. So, alcoholCH 3 OH sometimes called "wood alcohol" because it was once obtained by dry distillation of wood; the systematic name for this alcohol is methanol. The alkaloid morphine is named for its narcotic effect, but in this case the trivial name is the only one commonly used, since the systematic name is complex and cumbersome. Trivial names are often given to industrial products, especially in the pharmaceutical industry, where products are sold under proprietary names, and the same compound may be produced by different companies under different names. Quasi-systematic names are often used, which cannot adequately describe the structure of the compound without additional information. For example, the insecticide DDT is sometimes called dichlorodiphenyltrichloroethane, which is not enough to write a single structure for this compound, since the name does not say anything about the position of the chlorine atoms. The full name for the main active component is 2,2-di (4-chlorophenyl) -1,1,1-trichloroethane.
The basic rules for naming compounds according to the IUPAC system are given below:
1. Find the longest continuous chain of carbon atoms in a molecule. The name of the corresponding hydrocarbon is used as the basis for the name of the compound.
2. Atoms (other than hydrogen) and groups along this chain are given names, and these names are written before the name of the main hydrocarbon.
3. The carbon atoms of the main hydrocarbon chain are numbered sequentially, starting from the end selected so that the carbon atoms bearing the substituents are numbered as lowest.
4. The positions of the substituents are indicated by lokants - numbers before the names of substituents denoting the serial numbers of the carbon atoms to which they are attached.
5. If there are several identical groups, the prefix "di", "three", "tetra", "penta", "hexa", etc. is put before their name, indicating the number of groups present.
6. Double carbon-carbon bonds are indicated by the suffix "en" ("diene" if there are two of them, etc.), and triple - by the suffix "in" ("diyne" for two, etc.); when using these suffixes, the ending "an" is omitted. The position of the multiple bonds is designated by the serial numbers of the carbon atoms, similarly to how it is done for the substituents.
7. The whole name is written in one word.
Several examples illustrate these rules:

The name of such complex radicals asCH 3 CHCH 2 Cl in last example, is carried out according to the following rules:
1. A carbon atom with a "free" bond gets number 1ў. The longest carbon chain from this point on is numbered sequentially and used for the main name (ethane in this example).
2. With substituents along this chain proceed as described above when naming compounds.
3. The full name of a complex radical is enclosed in parentheses to avoid confusion with the numbers for the rest of the molecule.
IUPAC names and common names for several common complex radicals are given in Table. 2.
Cyclic hydrocarbons are named by adding the prefix "cyclo" to the name of the straight chain hydrocarbon. To indicate the position of substituents, ring atoms are numbered sequentially, starting with the main substituent (Table 3).

Note that in the last example the hydrocarbon is simply called benzene (and not 1,3,5-cyclohexatriene), and the corresponding radical - phenyl.
|
Table 3. NAMES OF THE MOST FREQUENTLY OCCURRING FUNCTIONAL (CHARACTERISTIC) GROUPS OF ORGANIC COMPOUNDS (GROUPS ARE LISTED FROM TOP TO DOWN IN ORDER OF DECREASING Seniority) |
|||
| Connections |
Groups |
Group names |
|
|
in the prefix |
in the suffix |
||
| Carboxylic acids | –COOH - (C) OOH a |
carboxy- – |
-carboxylic acid -oic acid |
| Sulfonic acids | –SO 3 H | sulfo- | -sulfonic acid (-sulfonic acid) |
| Amides | –CONH 2 - (C) ONH 2 a |
carbamoyl- – |
-carboxamide -amide |
| Nitriles | –Cє N - (C) є N a |
cyano- – |
-carbonitrile -nitrile |
| Aldehydes | –CH \u003d O - (C) H \u003d O a |
formyl- oxo |
-carbaldehyde -al |
| Ketones | - (C) \u003d O | oxo (keto) | -it |
| Alcohols, phenols | –OH | hydroxy- | -ol |
| Thiols | –SH | mercapto | -thiol |
| Amines | –NH 2 | amino | -amine |
| Ether b | –OAlk | alkoxy- | – |
| Halogen derivatives b | F, Cl, Br, I | fluoro-, chloro-, bromo-, iodo- | – |
| Nitrosocompounds b | –NO | nitroso- | – |
| Nitrocompounds b | –NO 2 | nitro- | – |
| Diazo compounds b | –N 2 | diazo- | – |
| Azides b | –N 3 | azido- | – |
|
AND The C atom in parentheses is considered part of the main carbon chain, not a functional group (CH 3 COOH - ethanic, methane carbonic, acetic acid). |
|||
More complex cyclic compounds are usually given trivial names and numbering systems. Compounds of this type include polycyclic aromatic hydrocarbons (in which the benzene rings are linked by two common atoms) and heterocyclic compounds (in which the rings contain heteroatoms). The most important cyclic systems and their numbering are given in table. 4. Note that in heterocycles, the numbering begins with a heteroatom and is done so that other heteroatoms are given the lowest numbers. The naming of substituents on these rings follows the basic IUPAC rules given above.

The most widely used IUPAC rules for constructing the names of organic compounds recommend the use of a substituent nomenclature. The general scheme of such names: 1)prefixes - side chains, then minor functions (cm ... tab. 3) in alphabetical order; 2)root - the main chain or cycle; 3)suffixes - multiple connections, the main function. for instance

Geometric isomerism is indicated by the prefixescis - and trance - (see above ).
Optical isomerism is indicated by the symbolsD-, L- or meso - before the name of the compound to indicate the row to which it belongs. Other systems are used less frequently. The direction of rotation of plane-polarized light is often indicated by a (+) sign for dextrorotatory and a (-) sign for levorotatory isomers.
For acids, in addition to their systematic names, trivial names are widely used in the scientific literature. Some important organic acids are listed below (Tables 5 and 6).
At the initial stage of the development of chemistry, the nature of organic substances was not fully understood, so they were given trivial names associated with their properties ( glycine - sweet) or sources of their receipt ( wine alcohol). Well-established trivial names are allowed for use by the IUPAC rules.
Trivial names are the simplest saturated hydrocarbons, and they underlie the names of all other classes of acyclic compounds, and the names of radicals are used in IUPAC nomenclatures and rational nomenclature.
The names of n-alkanes C n H 2n + 2
| Alkane formula | Name |
| CH 4 | Methane |
| C 2 H 6 | Ethane |
| C 3 H 8 | Propane |
| C 4 H 10 | Butane |
| C 5 H 12 | Pentane |
| C 6 H 14 | Hexane |
| C 7 H 16 | Heptane |
| C 8 H 18 | Octane |
| C 9 H 20 | Nonan |
| C 10 H 22 | Dean |
| S 11 N 24 | Undecane |
| C 12 H 26 | Dodecane |
| C 13 H 28 | Tridecan |
| C 14 -C 19 | Tetradecane, etc. |
| C 20 N 42 | Eicosan |
| C 21 H 44 | Geneicosan |
| S 22 N 46 | Dokosan |
| C 23 N 48 | Tricosan |
| C 24 -C 29 | Tetracosan, etc. |
| C 30 N 62 | Triacontan |
| C 31 H 64 | Gentriacontan |
| C 32 -C 39 | Dotriakontan, etc. |
| C 40 N 82 | Tetrakontan |
| S 41 N 84 | Gentetrakontan, etc. |
The names of some monovalent radicals
| Alkane formula and its name | Alkyl formula | Alkyl name | |
| trivial | systematic | ||
| Drank | Drank | ||
| Isopropyl | 1-methylethyl | ||
| Butyl | Butyl | ||
 |
Deut. butyl | 1-methylpropyl | |
|
Isobutyl |
 |
Isobutyl | 2-methylpropyl |
| Tret. butyl | 1,1-dimethylethyl | ||
|
Isopentane |
 |
Isopentyl | 3-methylbutyl |
 |
Deut. Isopentyl | 1,2-dimethylpropyl | |
 |
Tret. pentyl | 1,1-dimethylpropyl | |
 |
— | 2-methylbutyl | |
|
Neopentane |
 |
Neopentyl | 2,2-dimethylpropyl |
The names of some unsaturated radicals
Rational nomenclature
The rational name of an organic compound is based on the name of the prototype, the hydrogen atoms of which are replaced by radicals. As a rule, the simplest member of the homologous series acts as a prototype.
| Class | Prototype | The rule | Example |
| Alkanes | Methane |
the most branched carbon atom is chosen for methane carbon adjacent radicals should be the least complex the presence of several identical radicals is indicated by the corresponding multiplying prefix "di-", "tri-", "tetra-" |
Methyl ethyl isopropyl methane |
| Unsaturated hydrocarbons | Ethylene, acetylene | To indicate the location of the substituents, the C-atoms of the prototype are designated by the Greek letters α and β or the numbers 1 and 2. |
α-ethyl-β-tert-butyl ethylene |
| Alcohols | Carbinol |
Isopropenyl tert-butyl carbinol |
|
| Aldehydes | Acetaldehyde |
Vinyl isopropyl acetaldehyde |
|
| Ketones | Ketone |
Methyl propargyl ketone |
|
| Carboxylic acids | Acetic acid |
Isopropyl ethynyl acetic acid |
Radical functional nomenclature
- used to name simple mono- and bifunctional compounds
- highlights the main chemical feature connections
Rules for constructing a name based on a radical functional nomenclature:
- select the highest characteristic group (indicated by the name of the functional class), then add the name of the organic radical
- the name of the functional class is determined by the senior characteristic group, other groups are indicated by prefixes
- in compounds with multivalent characteristic groups, various radicals are listed in alphabetical order
- identical radicals are denoted by multiplying prefixes (di-, tri-)
Functional class names used in the radical functional nomenclature (in descending order of precedence)
| Group | Functional class name |
| X-derivatives of acids RCO-X, RSO 2 -X, etc. | X: fluoride, chloride, bromide, iodide, cyanide, azide; sulfur analogues, selenium analogues |
| -CN, -NC | Cyanide, isocyanide |
| \u003e CO | Ketone, then S - and then Se analogues |
| -OH | Alcohol, then S - and then Se-analogues |
| -O-OH | Hydroperoxide |
| -O- | Ether or oxide (oxide) |
| \u003e S,\u003e SO,\u003e SO 2 | Sulfide, sulfoxide, sulfone |
| \u003e Se. \u003e SeO,\u003e SeO 2 | Selenide, selenoxide, selenone |
| -F, -Cl, -Br, -I | Fluoride, chloride, bromide, iodide |
| -N 3 | Azide |
Examples of names of compounds according to the radical functional nomenclature

isobutyl alcohol
![]()
Vinyl chloride
![]()
Ethyl cyanide

Propionyl chloride

benzyl ethyl ketone

methyldiethylamine

isopropyl methyl sulfide

Isobutylethyl ether

Dimethyl sulfoxide

Second butyl chloride

2-Bromobutyl alcohol

3-Hydroxyisopropyl ketone
Substitute nomenclature
- is based on the principle of substitution in the structure that serves as the basis of the name, hydrogen atoms with various substituents
- the name is constructed as a complex word, consisting of a root (name of the main chain), suffixes reflecting the degree of its unsaturation (en, in), prefixes and endings characterizing the number and nature of substituents, indicating the numbers (locants) of their location
Saturated hydrocarbons
- Choose the longest chain of carbon atoms (main chain); if in a branched hydrocarbon there are chains of equal length, then the most branched
- The chain is numbered; the direction of numbering is chosen so that the locants (numbers indicating the position of the substituents) are the smallest.
- The name of the hydrocarbon with a number corresponding to the length of the main chain is added to the locant with the name of the substituent. In this case, the following rules must be observed:
- substitutes are listed in alphabetical order
- repeated identical substituents are named with the addition of multiplying prefixes (di-, tri-, tetra-, etc.). Prefixes do not affect alphabetical order
- numbers are separated from letters by a hyphen, and from each other by a comma
- each deputy has its own locant

2,3,5-Trimethylmethine-4-propylheptane

2,2,4-Trimethylpentane
Unsaturated hydrocarbons
- the name of unsaturated hydrocarbons with one double bond is derived from the name of the corresponding alkane by replacing the suffix "an" with "en"
- the longest carbon chain containing a double bond is chosen as the main one
- the chain is numbered so that the double bond gets the smallest ordinal number
- the name of hydrocarbons with a triple bond is formed from the names of the corresponding alkanes, replacing the suffix "an" by "in", and then by analogy with alkenes
- unsaturated hydrocarbons with two double bonds get the suffix "diene", with three - "triene", with two triple bonds - "diyne", etc.
- if there is both a double and a triple bond in the compound, add the suffix "enin"
- a double bond is considered older than a triple bond and gets a lower number

3-Isopropylpentene-1-yn-4
Monofunctional derivatives of hydrocarbons
Two types of characteristic groups:
- are designated as prefixes and are listed in alphabetical order simultaneously with hydrocarbon radicals
- can be included in a substitute name either in the form of a suffix or in the form of a prefix, depending on their relative precedence
Characteristic groups designated in the substituent, nomenclature only in prefixes
Designation of the most important groups in prefixes and suffixes in descending order of precedence (the carbon atom in parentheses is an integral part of the main carbon chain)
| Classes | Formula | Prefix designation | Suffix designation |
| Cation | -they are about- | -they are about- | -onium |
| Carboxyl | -COOH - (C) (\u003d O), OH |
-carboxy- — |
-carboxylic acid -oic acid |
| Sulfonic acid | -SO 3 H | -sulfo | -sulfonic acid |
| Esters | -COOR - (C) (\u003d O), R |
R-hydroxycarbonyl — |
R ... carboxylate R ... oat |
| Nitriles | -C≡N - (C) ≡N |
cyano- — |
-carbonitrile -nitrile |
| Aldehydes | -CHO | formyl | -carbaldehyde |
| - (C) H (\u003d O) | oxo | -al | |
| Ketones | (C \u003d O) | oxo | -it |
| Alcohols | -OH | hydroxy- | -ol |
| Phenols | -OH | hydroxy- | — |
| Thiols | -SH | mercapto | -thiol |
| Hydroperoxides | -O-OH | hydroperoxy- | — |
| Amines | -NH 2 | amino | -amine |
| Imines | \u003d NH | imino | -min |
| Ethers | -OR | R-hydroxy | — |
| Sulphides | -SR | R-thio- | — |
| Peroxides | -O-OR | R-dioxy- | — |
- in monofunctional compounds, characteristic groups of the second kind are indicated only by suffixes
- the chain is numbered so that the substituent listed alphabetically first gets the smallest number
- if the chain is not saturated, then multiple bonds are preferred for numbering

2-Methyl-3-chlorobutane

4-Bromo-2-pentene

Propantriol

2-Methylbutenal

5-Methyl-3-heptene-2,6-dione

4-Bromo-2-heptene-5-indioic acid
Polyfunctional compounds
1. Selection of the senior functional group
Of all the functional groups, the oldest is chosen - this group is indicated in the suffix, the rest are placed in the form of prefixes.
2. Main circuit selection
- The main circuit must contain the maximum number of senior groups
- The main chain should have the maximum number of double and triple bonds; with the same quantity, preference is given to double
- The main circuit must have the maximum length
- The main chain should have the maximum number of substituents indicated by prefixes
3. Chain numbering
The beginning and direction of numbering are chosen in such a way that the smallest digital indices receive the following structural elements of the connection (in the given order):
- major groups indicated by the suffix
- total unsaturation (i.e. the sum of double and triple bonds)
- double bonds
- triple bonds
- atoms or groups specified in prefixes
- prefixes in the listed order (alphabetically)
4. Composing the name of the compound
Prefixes are arranged alphabetically. Complex radicals form a single prefix, which is included in the alphabetical order according to the first letter of the name. In the case of identical prefixes with different digital locants, the prefix with the lowest locant is put first. Italics (for example, trans, sec., Sym.) In alphabetical order are not counted.

2-Pentene-2,4-disulfonic acid

2-Methyl-3-cyanopropanoic acid
Organic chemistry is the chemistry of carbon compounds, or, in other words, the chemistry of hydrocarbons and their derivatives. What is the classification and nomenclature of organic compounds?
What are organic compounds?
According to their composition, organic compounds are divided into classes - hydrocarbons and functional derivatives of hydrocarbons.
Hydrocarbons are organic compounds containing only carbon and hydrogen atoms (based on a chain of carbon atoms).

Figure: 1. Table of hydrocarbons.
Functional derivatives of hydrocarbons have one or more functional (active) groups that contain atoms of other elements (except for carbon and hydrogen) and determine the properties of this class of compounds. The functional groups include atoms of elements such as oxygen, nitrogen, sulfur. The main classes of organic compounds are characterized by the type of functional groups.
According to the shape of the carbon chain, organic compounds are divided into compounds of normal and isostructural structures, as well as compounds with an open carbon chain (acyclic) and with a closed carbon chain (cyclic).
Compounds of normal structure have a carbon chain without branching, and compounds of isostructure have branches in a carbon chain

Figure: 2. Types of carbon chains.
Type chemical bond organic compounds between carbon atoms are divided into saturated (limiting) and unsaturated (unsaturated). Saturated ones contain only simple carbon-carbon bonds, and unsaturated ones contain at least one multiple bond.
Compounds with an open chain - saturated and unsaturated - are called fatty compounds., Or aliphatic.
Cyclic compounds (saturated and unsaturated) are called alicyclic.
There are compounds with a special type of connection, which are called aromatic
Nomenclature of organic compounds
At present, organic compounds are named according to the rules of the International Systematic Nomenclature. For compounds common in everyday life and industry, especially natural ones, it is used trivial nomenclature, including historical names. For some, especially monofunctional, compounds, a type of MCH is used - a radical-functional nomenclature.
Basic principles of composing the name of a compound according to the SIT:
- the molecule is regarded as a derivative saturated hydrocarbon;
- the longest carbon chain containing the functional group or multiple bond, if any, is selected in the molecule. the chain is referred to as the corresponding saturated hydrocarbon;
- the main chain is numbered from that end to which the older group in the molecule is closer;
- if there is a multiple bond in the main chain, then the ending -an in the name of the limiting hydrocarbon changes to the corresponding one;
- if there is a functional group in the main chain, then the corresponding ending is added to the name of the main chain;
- before the name of the main chain, list the names of radicals that are not included in the main chain, but are associated with it, with the addition of one locant for each radical.