Teacher:
Educational institution: professional lyceum of the metro of St. Petersburg
Academic discipline: chemistry
Theme: "Oxygen-containing and nitrogen-containing organic compounds"
The target audience: 1 course
Lesson type: generalization of material, 1 acad. hour.
Lesson objectives:
Knowledge: know the formulas and properties of oxygen-containing and nitrogen-containing organic substances
Understanding:understand the dependence of the properties of substances on the structure of the molecule, on the functional group
Application: use information about the properties of substances to draw up equations of chemical reactions.
Analysis: analyze the mutual influence of groups of atoms in molecules of organic substances.
Synthesis:generalize information about the properties of organic substances in the form of a chain of transformations
Rating: self-assessment according to the proposed headings.
Equipment: interactive whiteboard, multimedia presentation.
Lesson plan:
1. Org. moment
2. Repetition of what was previously learned.
3. Students' speeches.
4. Self-determination of students by levels of self-esteem.
5. Independent work students.
6. Summing up the results according to the criterion-oriented system.
7. Homework.
During the classes
1. Organizing time.
Group building, group leader report on the number of students present.
2. Repetition of previously learned
Information about functional groups, classes of oxygen-containing and nitrogen-containing substances, about the simplest representatives of these classes with the use of an interactive whiteboard and multimedia presentation.
Which group of atoms, which is necessarily present in the molecules of substances of a given class, determines the chemical function of the substance, that is, its chemical properties?
Answer: functional group of atoms
Give a name to the functional group - OH
Answer: the hydroxyl group of atoms.
What class of substances does the hydroxyl group of atoms define?
Answer: Alcohols, if 1 group is OH, monohydric alcohol, if more than one group is OH, polyhydric alcohols.
Give the name to the functional group - DREAM. What class of substances does it define?
Answer: the aldehyde group determines the class of aldehydes.
Give the name of the functions to the group - DREAM. What class does she define?
Answer: the carboxyl group defines the class of carboxylic acids.
Name the functions to the group - NH2. What class does she define?
Answer: The amino group defines the class of amines or the class of amino acids.
We listen to students' messages with the presentation of multimedia presentations about the simplest representatives of various classes of oxygen-containing and nitrogen-containing substances.
3. Students' speeches.
Message 1.
Ethanol С2Н5ОН, class monohydric alcohols, functional group - hydroxyl group of atoms - OH. Qualitative reaction - interaction with copper (II) oxide with the formation of aldehyde. Chemical properties (select 2 reactions) - combustion and interaction with metals (Na).
Message 2.
Propanetriol (glycerol) C3H7 (OH) 3. Class - polyhydric alcohols, functional groups - several hydroxyl groups - OH. Qualitative reaction - interaction with copper (II) hydroxide. Chemical properties - interaction with sodium and with hydrogen halides.
Laboratory experience:
Pour about 1 ml of a solution of copper (II) sumorate into a test tube and add a little sodium hydroxide solution until a blue precipitate of copper (II) hydroxide is formed. Add glycerin solution dropwise to the resulting precipitate. Shake the mixture. We note the transformation of the blue precipitate into a blue solution.
(glycerin + Cu (OH) 2 ----- blue solution)
Message 3.
Phenol C6H5OH is the simplest representative of the phenol class.
The functional group is the hydroxyl group –OH. Qualitative reaction - the formation of a violet solution when interacting with iron (III) chloride or the formation of a white precipitate when interacting with bromine. Chemical properties: phenol is a weak acid, interacts in metals (Na) with alkalis (NaOH) and with bromine.
Message 4.
Ethanol or acetaldehyde CH3-COH Functional group - COH aldehyde group. Class - aldehydes. The qualitative reaction is the reaction of the "silver mirror". Chemical properties: reduction reaction and oxidation reaction.
Laboratory experience: demonstration experience.
In a test tube containing 1 ml of aldehyde ( water solution) add a few drops of ammonia solution of silver oxide. We heat the test tube. We observe the release of silver on the walls of the test tube, the glass surface becomes mirror-like.
Message 5.
Ethanic acid CH3-COOH (acetic acid). Class - carboxylic acids. Functional group - COOH carboxyl group. Qualitative reaction - the litmus indicator turns red.
Chemical properties: as any acid interacts with metals (Na), basic oxides (Na2O), alkalis (NaOH).
Laboratory experience:
Pour some acetic acid into a dry, clean test tube with a universal indicator. The indicator turns red.
Message 6.
Glucose C6H12O6. Class - carbohydrates. Functional groups: 5-OH and 1-COH, i.e. aldehydroalcohol. Qualitative reactions: interaction with copper hydroxide to form a blue solution. The reaction of the "silver mirror" with the release of silver on the walls of the test tube. Chemical properties: reduction to hexahedral alcohol, oxidation to gluconic acid, fermentation reaction.
Message 7.
Aniline C6H5-NH2.
Functional group - NH2 amino group. Class - amines. Qualitative reaction: interaction with bromine water with the formation of a white precipitate. Chemical properties: interaction with hydrochloric acid and with bromine.
Message 8.
Aminoethanic acid NH2-CH2-COOH or aminoacetic acid.
Class - amino acids. Functional groups: - NH2 amino group and - COOH carboxyl group. Chemical properties: AK - amphoteric compounds; - NH2 imparts basic properties, - COOH - acidic properties. Therefore, amino acids are able to combine with each other, forming protein molecules, and protein is the basis of life on our planet.
4. Self-determination of students by self-esteem levels.
Interactive whiteboard: students get acquainted with the self-assessment map of development in the lesson and mark their level.
1. I can determine the functional group and the simplest representative of the class of organic substances with the help of a teacher and a synopsis (6-7 points).
2. I can determine the functional group, the simplest representative of the class of organic substances, without the help of a teacher and without the help of a synopsis (8-10 points).
3. I can determine the qualitative reaction and chemical properties of a substance with the help of a teacher and a synopsis (11-14 points).
4. I can determine the qualitative reaction and chemical properties of a substance without the help of a teacher and without a synopsis (15-18 points).
Class | Functional groups | The simplest representative | Qualitative reactions | Chemical properties |
Monatomic alcohols | ||||
Polyhydric alcohols | ||||
Phenols | ||||
Aldehydes | ||||
Carboxylic acids | ||||
Carbohydrates | ||||
Amines | ||||
Amino acids |
Students are introduced to a criterion-based assessment system.
Criteria:
18 - 15 points - "excellent"
points - "good"
10 - 6 points - "satisfactory"
5 or less - "unsatisfactory"
5. Independent work of students.
6. Summing up the results according to a criterion-oriented system (announcing the number of points to students).
7. Homework:filling the table.
By clicking on the "Download archive" button, you will download the file you need for free.
Before downloading this file, remember those good abstracts, tests, term papers, theses, articles and other documents that are unclaimed on your computer. This is your work, it must participate in the development of society and benefit people. Find these works and submit to the knowledge base.
We and all students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
To download an archive with a document, in the field below, enter a five-digit number and click the "Download archive" button
Similar documents
- - primary amines YMN 2;
- - secondary amines KI / NN;
- - tertiary amines KK "K" N.
Nomenclature of benzene derivatives, their varieties and methods of preparation, principles and directions practical use... Benzene structure and aromaticity. Hückel's rule and features of its application. Non-benzoic aromatic compounds.
abstract, added 08/05/2013
Aromatic hydrocarbons: general characteristics. Nomenclature and isomerism, physical and chemical properties of aromatic hydrocarbons. The mechanism of reactions of electrophilic and nucleophilic substitution in the aromatic series. The use of arenes, their toxicity.
abstract, added 12/11/2011
Alkanes are saturated hydrocarbons containing only simple carbon bonds. Alkane production: industrial method, nitration and oxidation. Hydrocarbons containing a double bond of carbon are alkenes or ethylene hydrocarbons. Diene hydrocarbons.
lecture added 02/05/2009
Unsaturated compounds with two double bonds in the molecule are diene hydrocarbons. The relationship between the structure of diene hydrocarbons and their properties. Methods for producing devinyl, isoprene, synthetic rubber. Organic halides and their classification.
lecture added on 02/19/2009
Structure, nomenclature of alkenes. Unsaturated hydrocarbons, the molecules of which contain one double C-C bond. Orbital hybridization. Image of the spatial structure of atoms. Spatial isomerism of the carbon skeleton. Physical properties of alkenes.
presentation added on 08/06/2015
Development of ideas about the organic origin of oil. Paraffinic, naphthenic and aromatic hydrocarbons. Saturation pressure of oil with gas. Crystallization temperature, turbidity, solidification. Difference in oil properties within an oil-bearing reservoir.
tutorial added 02/05/2014
The concept of alkanes (saturated hydrocarbons, paraffins, aliphatic compounds), their systematic and rational nomenclature... Chemical properties of alkanes, radical substitution and oxidation reactions. Obtaining and recovery of unsaturated hydrocarbons.
Organic substances are a class of compounds that contain carbon (excluding carbides, carbonates, carbon oxides and cyanides). The name "organic compounds" appeared at an early stage in the development of chemistry and speaks for itself scientists ... Wikipedia
One of the most important types organic compounds... They contain nitrogen. They contain in the molecule a bond carbon hydrogen and nitrogen carbon. The oil contains a nitrogen-containing heterocycle, pyridine. Nitrogen is part of proteins, nucleic acids and ... ... Wikipedia
Organogermanium compounds Organometallic compounds containing the bond "germanium carbon". Sometimes they are called any organic compounds containing germanium. The first organogermanic compound tetraethylgermane was ... ... Wikipedia
Organosilicon compounds are compounds in the molecules of which there is a direct silicon-carbon bond. Organosilicon compounds are sometimes called silicones, from the Latin name for silicon silicon. Organosilicon compounds ... ... Wikipedia
Organic compounds, organic matter class chemical compoundscontaining carbon (except for carbides, carbonic acid, carbonates, carbon oxides and cyanides). Contents 1 History 2 Classi ... Wikipedia
Organometallic compounds (MOCs) are organic compounds in the molecules of which there is a bond of a metal atom with a carbon atom / atoms. Contents 1 Types of organometallic compounds 2 ... Wikipedia
Organohalogen compounds are organic substances containing at least one C Hal carbon halogen bond. Organohalogen compounds, depending on the nature of the halogen, are subdivided into: Organofluorine compounds; ... ... Wikipedia
Organometallic compounds (MOCs) are organic compounds in the molecules of which there is a bond of a metal atom with a carbon atom / atoms. Contents 1 Types of organometallic compounds 2 Methods of obtaining ... Wikipedia
Organic compounds in which a tin-carbon bond is present can contain both divalent and tetravalent tin. Contents 1 Methods of synthesis 2 Types 3 ... Wikipedia
- (heterocycles) organic compounds containing rings, which, along with carbon, include atoms of other elements. Can be considered as carbocyclic compounds with hetero substituents (heteroatoms) in the ring. Most ... ... Wikipedia
Nitrogen, like oxygen, is often a part of organic substances, and its compounds are necessary for living organisms.
Compounds containing nitrogen are more diverse than those containing oxygen. This is due to the fact that nitrogen has a higher valence and at the same time it has three hybrid states, like a carbon atom. Connections with single communication C-S called amines, with a double bond C \u003d N - imines, with a triple bond C \u003d K - nitriles.
The essential difference between nitrogen and oxygen is that nitrogen can enter organic compounds both in a reduced and oxidized state. The electronegativity of nitrogen (x \u003d 3.0) is higher than that of carbon (x \u003d 2.5) and lower than that of oxygen (x \u003d 3.5). If nitrogen is bonded to carbon and hydrogen, then its oxidation state is -3. In compounds containing the nitro group -G) 2, nitrogen is bound with oxygen and carbon and is in the +3 oxidation state. Organic compounds with oxidized nitrogen contain an internal supply of oxidant. When there are several nitro groups in the molecule, the compound becomes explosive. This type of substance includes 2,4,6-trinitrotoluene (TNT).
Reduced nitrogen imparts to organic compounds the same properties as oxygen: polarity, basicity and acidity, ability
form hydrogen bonds. However, the polarity of nitrogen-containing compounds is lower and hydrogen bonds are weaker than those of oxygen-containing ones. Therefore, for some physical properties amines find themselves between hydrocarbons and alcohols. While all alcohols are liquids under normal conditions, some amines are gaseous:
Nitrogen able vr 3 -hybridization is a good electron pair donor. Therefore, as we already know, amines exhibit rather strong basic properties. To a lesser extent, the donor properties are expressed for nitrogen in the state of $ р 2 -hybridization. The acidic properties of nitrogen-containing organic compounds are much weaker than those of oxygen-containing ones. But with the participation of nitrogen electrons in conjugation with n-electrons and carbon, acidic properties manifest themselves.
One of the classes of nitrogen-containing substances - amines. This is the name of nitrogen-containing organic substances in which a nitrogen atom is combined with hydrocarbon radicals and the corresponding number of hydrogen atoms. Depending on the number of radicals, there are:
Note that the concepts of primary, secondary and tertiary amines do not coincide with the corresponding concepts for alcohols.
Distinguish homologous series limiting, unsaturated and aromatic amines. There is also a difference in terminology when comparing alcohols and amines. In aromatic alcohols, the hydroxyl group must be bonded to a carbon atom in the radical, not in the aromatic ring. In the case of nitrogen-containing compounds, a substance with an NH 2 group linked to an aromatic ring is also considered an amine.
Amines with low molecular weight are liquid or gaseous substances that are highly soluble in water. They have an unpleasant odor reminiscent of ammonia. The specific smell of fish is also associated with the presence of amines. Higher amines have the same features that were noted in alcohols and acids - the solubility in water decreases and surface activity appears.
Getting amines. One of the methods for producing amines is similar to the production of alcohols. These are reactions of halogenated hydrocarbons with ammonia, proceeding by the mechanism of nucleophilic substitution:
The amine here cannot be a direct reaction product, since the resulting hydrogen chloride reacts with it as with a base.
giving the amine salt. To highlight free amine, the resulting salt is treated with alkali:
The halogenated hydrocarbon reacts not only with ammonia, but also with primary amine... This forms a secondary amine, and at the next stage a tertiary amine:
Amines are also obtained by hydrogenation of nitriles:
Aromatic amines are obtained by reduction of nitro compounds. Metals are used as reducing agents in an acidic environment:
This aromatic amine is called aniline. The reduction reaction of nitro compounds was discovered by NN Zinin in 1842. In industry, nitrobenzene is reduced with hydrogen on a nickel catalyst at ~ 300 ° C. Aniline has become a very important intermediate product used for the production of dyes, polymers, drugs, etc. The world production of aniline is over 1 million tons per year.
Chemical properties of amines. Amines are among the substances capable of burning with the formation of C0 2, H 2 0 and nitrogen N 2.
As bases, amines are similar to ammonia, from which they are produced by replacing hydrogen with hydrocarbon radicals. These radicals affect the strength of the bases. The effect of inductive and mesomeric effects on basic properties is generally opposite to their effect on acidic properties. Limit alcohols by acidic properties weaker than water, and limiting amines in basic terms are stronger than ammonia; phenols are much stronger in acidic properties than alcohols, and aniline in basic properties is much weaker than saturated amines.
IN limiting amines +/- the effect of the radical increases the electron density on nitrogen, therefore the ability of nitrogen to donate an electron pair to form donor-acceptor bond... In aniline, the electron pair of nitrogen participates in conjugation with aromatic TT-electrons and becomes less available for the formation of a donor-acceptor bond. Therefore, the substances are arranged in the following row according to the weakening of the main properties:
limiting amines\u003e NH 3\u003e aromatic amines.
Example 22.15. In what direction is the equilibrium of the reaction between ethylamine and aniline hydrochloride shifted?
Decision. Ethylamine is a stronger base than aniline. Therefore, the equilibrium is shifted towards the formation of aniline:
Amines as bases react with metal ions to form complex compounds. The metal ion acts as an acceptor of the nitrogen electron pair, as in the case of reactions with ammonia. There are a lot of complex compounds of metals (i-block with various amines. When mixing solutions of copper sulfate and methylamine, an intensely colored solution of a purer blue hue is formed than in the case of a reaction with ammonia (paragraph 210):
diamines of the type rNh 2 CH 2 CH 2 1H 2 give more stable complexes than monoamines, since each molecule has two donor nitrogen atoms and is attached by two donor-acceptor bonds.
Primary amines by nitrous acid (or sodium nitrite in an acidic environment) deaminate turning into alcohols:
In primary and secondary amines, the amino hydrogen is replaced by hydrocarbon radicals in reactions with halogen derivatives (see the preparation of amines). An amine with an acid halide gives an acid amide in which there is a radical associated with nitrogen:
Tertiary amines add halogenated hydrocarbons to form tetra-substituted (quaternary) ammonium salts:
These are crystalline substances, readily soluble in water. Unlike conventional ammonium salts, they are not hydrolyzed or decomposed by alkalis.
In aniline and other aromatic amines, the NH 2 group exhibits a positive mesomeric effect, accelerating the reactions of electrophilic substitution in the aromatic radical. Aniline decolours bromine water, forming a white precipitate of tribromaniline.
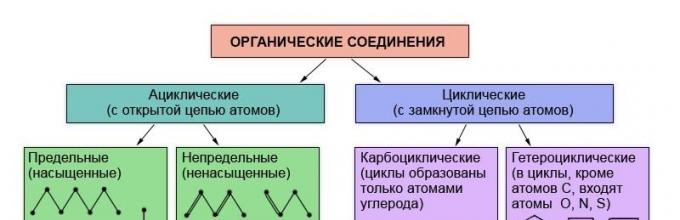
It is known that the properties of organic substances are determined by their composition and chemical structure... Therefore, it is not surprising that the classification of organic compounds is based on the theory of structure - the theory of L.M.Butlerov. Organic substances are classified according to the presence and order of connection of atoms in their molecules. The most durable and least changeable part of an organic substance molecule is its skeleton - a chain of carbon atoms. Depending on the order of joining of carbon atoms in this chain, substances are divided into acyclic, which do not contain closed chains of carbon atoms in molecules, and carbocyclic, containing such values \u200b\u200b(cycles) in molecules.
In addition to carbon and hydrogen atoms, molecules of organic substances can contain atoms and other chemical elements... Substances in the molecules of which these so-called heteroatoms are included in a closed chain are referred to as heterocyclic compounds.
Heteroatoms (oxygen, nitrogen, etc.) can be part of molecules and acyclic compounds, forming functional groups in them, for example, hydroxyl - OH, carbonyl, carboxyl, amino group -NH2.
Functional group - a group of atoms that determines the most characteristic chemical properties of a substance and its belonging to a certain class of compounds. 
Hydrocarbons- These are compounds consisting only of hydrogen and carbon atoms.
Depending on the structure of the carbon chain, organic compounds are divided into compounds with an open chain - acyclic (aliphatic) and cyclic - with a closed chain of atoms.
Cyclics are divided into two groups: carbocyclic compounds(the cycles are formed only by carbon atoms) and heterocyclic (the cycles also include other atoms, such as oxygen, nitrogen, sulfur).
Carbocyclic compounds, in turn, include two series of compounds: alicyclic and aromatic.
Aromatic compounds at the heart of the structure of molecules have flat carbon-containing cycles with a special closed system of p-electrons that form a common π-system (a single π-electron cloud). Aromaticity is also characteristic of many heterocyclic compounds.
All other carbocyclic compounds belong to the alicyclic series.
Both acyclic (aliphatic) and cyclic hydrocarbons can contain multiple (double or triple) bonds. Such hydrocarbons are called unsaturated (unsaturated), in contrast to limiting (saturated), containing only single bonds.
Saturated aliphatic hydrocarbons called alkanes, they have the general formula C n H 2 n +2, where n is the number of carbon atoms. Their old name is often used nowadays - paraffins.
Containing one double bond, got the name alkenes... They have the general formula C n H 2 n.
Unsaturated aliphatic hydrocarbonswith two double bonds called alkadienes
Unsaturated aliphatic hydrocarbonswith one triple bond called alkynes... Their general formula is C n H 2 n - 2.
Saturated alicyclic hydrocarbons - cycloalkanes, their general formula is C n H 2 n.
A special group of hydrocarbons aromatic, or arenas (with a closed common π-electron system) is known from the example of hydrocarbons with the general formula C n H 2 n -6.
Thus, if their molecules contain one or more replace hydrogen atoms with other atoms or groups of atoms (halogens, hydroxyl groups, amino groups, etc.), derivatives of hydrocarbons: halogen derivatives, oxygen-containing, nitrogen-containing and other organic compounds.
Halogen derivatives hydrocarbons can be considered as products of substitution in hydrocarbons of one or more hydrogen atoms by halogen atoms. In accordance with this, limiting and unsaturated mono-, di-, tri- (generally poly-) halogen derivatives can exist.
General formula of monohalogenated saturated hydrocarbons:
and the composition is expressed by the formula
C n H 2 n +1 Г,
where R is the residue from a saturated hydrocarbon (alkane), a hydrocarbon radical (this designation is used further when considering other classes of organic substances), G is a halogen atom (F, Cl, Br, I).
Alcohols - derivatives of hydrocarbons in which one or more hydrogen atoms are replaced by hydroxyl groups.
Alcohols are called monatomic, if they have one hydroxyl group, and limiting, if they are derivatives of alkanes.
The general formula of saturated monohydric alcohols:
and their composition is expressed by the general formula:
C n H 2 n +1 OH or C n H 2 n +2 O
Examples of polyhydric alcohols are known, i.e., having several hydroxyl groups.
Phenols - derivatives of aromatic hydrocarbons (benzene series), in which one or more hydrogen atoms in the benzene ring are replaced by hydroxyl groups.
The simplest representative with the formula C 6 H 5 OH is called phenol.
Aldehydes and ketones - derivatives of hydrocarbons containing a carbonyl group of atoms (carbonyl).
In the molecules of aldehydes, one bond of the carbonyl goes to compound with a hydrogen atom, the other - with a hydrocarbon radical.
In the case of ketones, the carbonyl group is bonded to two (generally different) radicals.
The composition of saturated aldehydes and ketones is expressed by the formula C n H 2l O.
Carboxylic acids - derivatives of hydrocarbons containing carboxyl groups (-COOH).
If there is one carboxyl group in the acid molecule, then the carboxylic acid is monobasic. General formula of saturated monobasic acids (R-COOH). Their composition is expressed by the formula C n H 2 n O 2.
Ethers are organic substances containing two hydrocarbon radicals connected by an oxygen atom: R-O-R or R 1 -O-R 2.
Radicals can be the same or different. The composition of ethers is expressed by the formula C n H 2 n +2 O
Esters - compounds formed by replacing the hydrogen atom of the carboxyl group in carboxylic acids to a hydrocarbon radical.
Nitro compounds - derivatives of hydrocarbons in which one or more hydrogen atoms are replaced by a nitro group —NO 2.
General formula for limit mononitro compounds:
and the composition is expressed by the general formula
C n H 2 n +1 NO 2.
Amines - compounds that are considered as derivatives of ammonia (NH 3), in which hydrogen atoms are replaced by hydrocarbon radicals.
Depending on the nature of the radical, amines can be aliphatic and aromatic.
Depending on the number of hydrogen atoms replaced by radicals, the following are distinguished:
Primary amines with the general formula: R-NH 2
Secondary - with the general formula: R 1 -NH-R 2
Tertiary - with a general formula:
In the particular case, for secondary and tertiary amines, the radicals can be the same.
Primary amines can also be considered as derivatives of hydrocarbons (alkanes) in which one hydrogen atom is replaced by an amino group —NH 2. The composition of the limiting primary amines is expressed by the formula C n H 2 n +3 N.
Amino acids contain two functional groups connected to a hydrocarbon radical: amino group —NH 2, and carboxyl —COOH.
The composition of the limiting amino acids containing one amino group and one carboxyl is expressed by the formula C n H 2 n +1 NO 2.
Other important organic compounds are known which have several different or identical functional groups, long linear chains linked to benzene rings. In such cases, a strict determination of the belonging of a substance to any particular class is impossible. These compounds are often isolated into specific groups of substances: carbohydrates, proteins, nucleic acids, antibiotics, alkaloids, etc.

For the name of organic compounds, 2 nomenclatures are used - rational and systematic (IUPAC) and trivial names.
IUPAC nomenclature compilation
1) The basis of the name of the compound is the root of the word denoting a saturated hydrocarbon with the same number of atoms as the main chain.
2) A suffix is \u200b\u200badded to the root characterizing the degree of saturation:
An (limiting, no multiple connections);
-en (in the presence of a double bond);
-in (in the presence of a triple bond).
If there are several multiple bonds, then the suffix indicates the number of such bonds (-dien, -triene, etc.), and after the suffix, the position of the multiple bond must be indicated in numbers, for example:
CH 3 –CH 2 –CH \u003d CH 2 CH 3 –CH \u003d CH – CH 3
butene-1 butene-2
CH 2 \u003d CH – CH \u003d CH 2
butadiene-1,3
Such groups as nitro-, halogens, hydrocarbon radicals not included in the main chain are carried out in the prefix. However, they are listed alphabetically. The position of the substitute is indicated by a number in front of the prefix.
The order of composing the name is as follows:
1. Find the longest chain of atoms C.
2. Consecutively number the carbon atoms of the main chain, starting from the end closest to the branch.
3. The name of the alkane is made up of the names of the side radicals listed in alphabetical order, indicating the position in the main chain, and the name of the main chain.
Nomenclature of some organic substances (trivial and international)