Module 1 Building, Properties and Protein Functions
Module 1 Building, Properties and Protein Functions
Module structure | Topics |
Modular unit 1. | 1.1. Structural organization of proteins. Stages of the formation of native protein conformation 1.2. Fundamentals of proteins. Drugs like ligands affecting the function of proteins 1.3. Denaturation of proteins and the possibility of their spontaneous renatitution |
Modular unit 2. | 1.4. Features of the structure and functioning of oligomeric proteins on the example of hemoglobin 1.5. Maintaining the native conformation of proteins in cell conditions 1.6. Variety of proteins. Family of proteins on the example of immunoglobulins 1.7. Physico-chemical properties of proteins and methods of their separation |
Modular unit 1 Structural organization of monomeric proteins and basics of their functioning
The objectives of the study are able to:
1. Use knowledge about the features of the structure of proteins and the dependence of protein functions from their structure to understand the development mechanisms of hereditary and acquired proteinopathies.
2. Explain the mechanisms of therapeutic action of some drugs as ligands interacting with proteins and varying their activity.
3. Use knowledge about the structure and conformational lability of proteins to understand their structural and functional instability and a tendency to denaturation in changing conditions.
4. Expand the use of denaturing agents as funds for sterilizing medical material and tools, as well as antiseptics.
Know:
1. Levels of the structural organization of proteins.
2. The value of the primary structure of proteins that defines their structural and functional manifold.
3. The mechanism of formation in the proteins of the active center and its specific interaction with the ligand underlying the functioning of proteins.
4. Examples of the effect of exogenous ligands (drugs, toxins, poisons) on the conformation and functional activity of proteins.
5. Causes and consequences of protein denaturation, denaturation factors.
6. Examples of using denaturing factors in medicine as antiseptics and means for sterilizing medical instruments.
Topic 1.1. Structural organization of proteins. Stages of native formation
Conformations of proteins
Proteins are polymer molecules whose monomers are only 20 α-amino acids. The set and order of the compound of amino acids in protein is determined by the structure of genes in DNA of individuals. Each protein in accordance with its specific structure performs the feature characteristic of it. The set of proteins of this organism determines its phenotypic features, as well as the presence of hereditary diseases or predisposition to their development.
1. Amino acids included in proteins. Peptide connection.Proteins - polymers built from monomers - 20 α-amino acids, the total formula
Amino acids differ in structure, sizes, physicochemical properties of radicals attached to the α-carbon atom. Functional groups of amino acids determine the features of the properties of different α-amino acids. The radicals found in α-amino acids can be divided into several groups:
Prolineunlike other 19 monomers of proteins, not an amino acid, but imino acid, radical in the proline is associated both with an α-carbon atom and with the imino group
 Amino acids differ in solubility in water.This is due to the ability of radicals to interact with water (hydrate).
Amino acids differ in solubility in water.This is due to the ability of radicals to interact with water (hydrate).
TO hydrophilicradicals containing anionic, cationic and polar uncharged functional groups.
TO hydrophobicradicals containing methyl groups, aliphatic chains or cycles.
2. Peptide bonds connect amino acids in peptides.In the synthesis of a peptide, an α-carboxyl group of one amino acid interacts with an α-amino group of another amino acid to form peptide Communication:
Proteins are polypeptides, i.e. Linear polymers α-amino acids connected peptide connections (Fig. 1.1.)
 Fig. 1.1. Terms used in describing the structure of peptides
Fig. 1.1. Terms used in describing the structure of peptides
Amino acid monomers included in the polypeptides are called amino acid residues.Chain of repeating groups - NH-CH-CO- forms peptide core.Amino acid residue having a free α-amino group is called N-terminal, and having a free α-carboxyl group - C-terminal. Peptides are recorded and read from the N-terminus to the C-End.
The peptide bond formed by the proline imino group differs from other peptide bonds: there is no hydrogen in the nitrogen atom of the peptide group,
instead, it has a connection with a radical, as a result, one side of the cycle is included in the peptide cable:
 Peptides are varying by amino acid composition, the amount of amino acids and the order of the compound of amino acids, for example, Serma-de-GIS and GIS-GIS-Ala-Seri-Series are two different peptides.
Peptides are varying by amino acid composition, the amount of amino acids and the order of the compound of amino acids, for example, Serma-de-GIS and GIS-GIS-Ala-Seri-Series are two different peptides.
Peptide ties are very durable, and rigid conditions are required for their chemical nefermental hydrolysis: the analyzed protein is hydrolyzed in concentrated hydrochloric acid at a temperature of about 110 ° for 24 hours. In a lively cell, peptide ties can be broken up with proteolytic enzymescalled proteasesor peptidhydrolases.
3. Primary protein structure.Amino acid residues in peptide chains of different proteins alternate not randomly, but are located in a certain order. Linear sequence or order of alternation of amino acid residues in the polypeptide chain is called primary protein structure.
The primary structure of each individual protein is encoded in the DNA molecule (in a plot called gene) and is implemented during transcription (rewriting information on mRNA) and translation (synthesis of primary protein structure). Consequently, the primary structure of the individuals of an individual person - the information determining the features of the structure of proteins of this body, which depends on the function of the existing proteins from parents to children, which depends on the function of the proteins (Fig. 1.2.).
 Fig. 1.2. The relationship between the genotype and the conformation of proteins synthesized in the body of an individual
Fig. 1.2. The relationship between the genotype and the conformation of proteins synthesized in the body of an individual
Each of the approximately 100,000 individual proteins in the human body has uniqueprimary structure. In the molecules of one type of protein (for example, albumin), the same album of amino acid residues, which distinguishes albumin from any other individual protein.
The sequence of amino acid residues in the peptide chain can be viewed as a form of information recording. This information determines the spatial laying of a linear peptide chain into a more compact three-dimensional structure called conformationsquirrel. The process of forming functionally active conformation of the protein is called folding.
4. Conformation of proteins.The free rotation in the peptide isolation is possible between the nitrogen atom of the peptide group and the adjacent α-carbon atom, as well as between the α-carbon atom and carbon carbonyl group. Due to the interaction of functional groups of amino acid residues, the primary structure of proteins can acquire more complex spatial structures. In globular proteins distinguish two main levels of laying of the conformation of peptide chains: secondaryand tertiary structures.
Secondary structure of proteins- This is a spatial structure that is formed as a result of the formation of hydrogen bonds between functional groups -C \u003d O and - NH-peptide island. In this case, the peptide chain may acquire regular structures of two types: α-spiraland β-structures.
IN α-spiralhydrogen bonds are formed between the oxygen atom of the carbonyl group and the hydrogen of amide nitrogen 4th of the amino acid; Side chains of amino acid residues
they are located along the periphery of the spiral, without participating in the formation of the secondary structure (Fig. 1.3.).
Volumetric radicals or radicals carrying identical charges prevent the formation of α-helix. The residue of the proline having a ring structure interrupts the α-spiral, since due to the lack of hydrogen at the nitrogen atom in the peptide chain it is impossible to form a hydrogen bond. The relationship between nitrogen and the α-carbon atom is part of the proline cycle, so the peptide of the core in this place becomes bending.
β-structureit is formed between the linear regions of the peptide island of one polypeptide chain, forming folded structures. Polypeptide chains or parts them can form parallelor anti-parallel β-structures.In the first case, the N- and C-ends of the interacting peptide chains coincide, and in the second - they have the opposite direction (Fig. 1.4).
Fig. 1.3. Secondary protein structure - α-spiral
 Fig. 1.4. Parallel and anti-parallel β-folded structures
Fig. 1.4. Parallel and anti-parallel β-folded structures
β-structures are marked by wide arrows: a - anti-parallel β-structure. B - parallel β-folded structures
In some, the proteins of β-structure can be formed by the formation of hydrogen bonds between the atoms of the peptide island of different polypeptide chains.
In proteins are also found areas with irregular secondarythe structure to which are bends, loops, turns of the polypeptide island. They are often located in places where the direction of the peptide chain changes, for example, when forming a parallel β-folded structure.
According to the presence of α-helix and β-structures, globular proteins can be divided into four categories.
Fig. 1.5. The secondary structure of myoglobin (A) and the β-chain of hemoglobin (b) containing eight α-spirals

 Fig. 1.6. Secondary structure of triosophosphatisomerase and piruvatkinase domain
Fig. 1.6. Secondary structure of triosophosphatisomerase and piruvatkinase domain
 Fig. 1.7. Secondary structure of the constant domain of immunoglobulin (a) and the enzyme superoxiddismutase (b)
Fig. 1.7. Secondary structure of the constant domain of immunoglobulin (a) and the enzyme superoxiddismutase (b)
IN fourth categoryincluded proteins that have an insignificant number of regular secondary structures in their composition. Such proteins include small, rich in cysteine \u200b\u200bproteins or metalloproteins.
Tertiary protein structure- The type of conformation, formed by the interactions between the amino acid radicals, which can be at a considerable distance from each other in the peptide chain. Most proteins at the same time form a spatial structure resembling globule (globular proteins).
Since hydrophobic amino acid radicals tend to combine with the help of the so-called hydrophobic interactionsand intermolecular van der Waals forces, a dense hydrophobic core is formed inside the protein globule. Hydrophilic ionized and non-ionized radicals are mainly located on the surface of the protein and determine its solubility in water.
 Fig. 1.8. Types of connections arising between amino acid radicals in the formation of the tertiary protein structure
Fig. 1.8. Types of connections arising between amino acid radicals in the formation of the tertiary protein structure
1 - ion communication- arises between positively and negatively charged functional groups;
2 - hydrogen communications- arises between hydrophilic uncharged and any other hydrophilic group;
3 - hydrophobic interactions- arise between hydrophobic radicals;
4 - disulfide communications- formed by oxidation of SH-groups of cysteine \u200b\u200bresidues and their interaction with each other
Hydrophilic amino acid residues that found themselves inside the hydrophobic kernel can interact with each other with ionicand hydrogen ties(Fig. 1.8).
Ionic and hydrogen bonds, as well as hydrophobic interactions relate to the number of weak: their energy slightly exceeds the energy of the thermal motion of molecules at room temperature. The conformation of the protein is supported by the occurrence of many of such weak ties. Since the atoms from which protein consists are in constant motion, it is possible to break down the weak bonds and the formation of others, which leads to a small movement of individual sections of the polypeptide chain. This property of proteins change the conformation as a result of the breaking of some and the formation of other weak ties is called conformational lability.
In the human body, systems operate supporting homeostasis- Constancy interior environment In certain valid values \u200b\u200bfor a healthy organism. Under the conditions of homeostasis, small changes in the conformation do not violate the overall structure and the function of proteins. Functionally active protein conformation is called native conformation.Change in the inner medium (for example, glucose concentrations, Ca ions, protons, etc.) leads to a change in conformation and impaired protein functions.
The tertiary structure of some proteins is stabilized disulfide bondsformed by the interaction of -shh groups of two residues
 Fig. 1.9. Formation of disulfide bond in the protein molecule
Fig. 1.9. Formation of disulfide bond in the protein molecule
cysteine \u200b\u200b(Fig. 1.9). Most intracellular proteins do not have in the tertiary structure of covalent disulfide bonds. The presence is characteristic of protein secreted cells, which ensures their greater stability in extracellular conditions. Thus, disulfide bonds are available in insulin and immunoglobulin molecules.
Insulin- protein hormone, synthesized in the β-cells of the pancreas and secreted in blood in response to an increase in blood glucose concentration. In insulin structure, there are two disulfide bonds connecting polypeptide A- and B-chains, and one disulfide bond inside the A-chain (Fig. 1.10).
 Fig. 1.10. Disulfide bonds in insulin structure
Fig. 1.10. Disulfide bonds in insulin structure
5. Superventor protein structure.In different primary structure and functions, proteins are sometimes revealed similar combinations and interpretation of secondary structures,which are called a super-warning structure. It occupies an intermediate position between secondary and tertiary structures, since this is a specific combination of elements of the secondary structure in the formation of the tertiary protein structure. Supervogenous structures have specific names, such as "α-spiral-rotate-a-spiral", "leucin zipper", "zinc fingers", etc. Such super-functional structures are characteristic of DNA-binding proteins.
"Leucin zipper".This type of super-standard structure is used to connect two proteins. On the surface of the interacting proteins there are α-spiral sections containing at least four leucine residues. Leucine residues in α-spirals are located in six amino acids one from the other. Since each α-helix coil contains 3.6 amino acid residues, leucine radicals are on the surface of each second turn. Leucine residues of α-helix of one protein can interact with leucine residues of another protein (hydrophobic interactions), connecting them together (Fig. 1.11.). Many DNA binding proteins function as part of oligomeric complexes, where individual subunits are associated with each other "leucine clasps".
 Fig. 1.11. "Leucin zipper" between α-spiral sections of two proteins
Fig. 1.11. "Leucin zipper" between α-spiral sections of two proteins
An example of such proteins can serve as histones. Histons- nuclear proteins which included a large number of Positively charged amino acids - arginine and lysine (up to 80%). Histon molecules are combined into oligomeric complexes containing eight monomers using "leucine fasteners", despite the significant charge of these molecules.
"Zinc finger"- A variant of the super-standard structure characteristic of DNA-binding proteins has the form of an elongated fragment on the protein surface and contains about 20 amino acid residues (Fig. 1.12). The form of the "elongated finger" maintains a zinc atom associated with the radicals of four amino acids - two of the remains of cysteine \u200b\u200band two - histidine. In some cases, instead of the residues of histidine are the remains of cysteine. Two closely lying cysteine \u200b\u200bresidue are separated from two other residues of hyicyli cycoselitivity consisting of about 12 amino acid residues. This section of the protein forms an α-spiral, the radicals of which can specifically bind to the regulatory sections of the large groove of DNA. Private binding specificity
 Fig. 1.12. The primary structure of the DNA-binding proteins section forming the "zinc finger" structure (letters are indicated by amino acids included in this structure)
Fig. 1.12. The primary structure of the DNA-binding proteins section forming the "zinc finger" structure (letters are indicated by amino acids included in this structure)
regulatory DNA binding protein depends on the sequence of amino acid residues located in the area of \u200b\u200bthe "zinc finger". Such structures contain, in particular, the receptors of steroid hormones involved in transcription regulation (reading information from DNA on RNA).
Topic 1.2. Fundamentals of proteins. Drugs like ligands affecting the function of proteins
1. The active center of the protein and its interaction with the ligand.In the process of forming the tertiary structure on the surface of the functionally active protein, it is usually in the recess, a plot formed by the amino acid radicals, far standing from each other in the primary structure. This plot having a unique structure for this protein and capable of specific interact with a specific molecule or group of similar molecules is called a protein binding center with a ligand or an active center. Ligands are called molecules that interact with proteins.
High specificitythe interaction of protein with ligand is ensured by the complementarity of the structure of the active center of the structure of the ligand.
Complementation- It is the spatial and chemical compliance of interacting surfaces. The active center should not only spatially comply with the ligand included in it, but also between the functional groups of radicals included in the active center, and the ligand must be formed by communication (ionic, hydrogen, as well as hydrophobic interactions) that hold the ligand in the active center (Fig. 1.13 ).
 Fig. 1.13. Complementary interaction of protein with ligand
Fig. 1.13. Complementary interaction of protein with ligand
Some ligands, joining the active center of the protein, perform an auxiliary role in the functioning of proteins. Such ligands are called cofactors, and proteins that have a non-peculiar part - complex proteins(Unlike simple proteins consisting of protein only). Non-worn part, firmly connected to protein, is called prosthetic group.For example, the composition of myoglobin, hemoglobin and cytochromes contains a firmly attached to the active center a prosthetic group - gem containing iron ion. Complex proteins containing gem are called hemoproteins.
When connected to proteins of specific ligands, the function of these proteins is manifested. So, albumin is the most important plasma protein - manifests its transport function, connecting hydrophobic ligands to the active center, such as fatty acids, bilirubin, some medicines, etc. (Fig. 1.14)
Ligands interacting with the three-dimensional structure of the peptide chain may not only be low molecular weight organic and inorganic molecules, but also macromolecules:
DNA (examples considered above with DNA-binding proteins);
Polysaccharides;
 Fig. 1.14. Interconnection of the genotype and phenotype
Fig. 1.14. Interconnection of the genotype and phenotype
The unique primary structure of human proteins encoded in the DNA molecule in the cells is realized in the form of a unique conformation, the structure of the active center and the functions of proteins
In these cases, the protein recognizes a certain area of \u200b\u200bligand, commensuous and complementary binding center. So on the surface of hepatocytes there are proteins receptors to hormone insulin, which also has protein structure. The interaction of insulin with the receptor causes a change in its conformation and activation of signaling systems leading to stamps in nutrocytes of nutrients after meals.
In this way, the basis of the functioning of proteins is the specific interaction of the active center of the protein with the ligand.
2. The domain structure and its role in the functioning of proteins.Long polypeptide chains of globular proteins are often folded in several compact, relatively independent areas. They have an independent tertiary structure resembling such in globular proteins, and are called domains.Thanks to the domain structure of proteins, they are easier formed tertiary structure.
In the domain proteins, binding centers with ligand are often located between the domains. Thus, trypsin is a proteolytic enzyme that is produced by an exocrine part of the pancreas and is necessary for digesting food proteins. It has a two-dimensional structure, and the TRIPSIN binding center with its ligand - food protein - is located in the groove between the two domains. The active center creates the conditions necessary for the effective binding of a specific section of the food protein and hydrolysis of its peptide bonds.
Different domains in protein when interacting the active center with ligand can be moved relative to each other (Fig. 1.15).
Hexokinas- enzyme catalyzing glucose phosphorylation using ATP. The active center of the enzyme is located in the cleft between the two domains. When binding hexochinases with glucose, the surrounding domains are closed and the substrate turns out to be a "trap", where phosphorylation occurs (see Fig. 1.15).
 Fig. 1.15. Binding of domains of hexochinases with glucose
Fig. 1.15. Binding of domains of hexochinases with glucose
Some domain proteins perform independent functions, binding to various ligands. Such proteins are called multifunctional.
3. Medicines - ligands affecting the function of proteins.The interaction of proteins with ligands is specifically. However, due to the conformational lability of the protein and its active center, it is possible to choose another substance that could also interact with the protein in the active center or other section of the molecule.
Substance, according to the structure similar to the natural ligand, is called structural analogue of Ligandaor non-ligand. It also interacts with the protein in the active center. Structural analogue of the ligand can how to enhance the protein function (agonist),so reduce her (antagonist).Ligand and its structural analogs compete with each other for binding to protein in one center. Such substances are called competitive modulators(regulators) protein functions. Many drugs act as protein inhibitors. Some of them are obtained by a chemical modification of natural ligands. Inhibitors of protein functions may be medicines and poisons.
Atropine is a competitive inhibitor of m-cholinoreceptors.Acetylcholine is a neurotransmitter of the transmission of a nervous pulse through cholinergic synapses. To carry out the excitation, acetylcholine elected to the synaptic slot should interact with the protein - a postsynaptic membrane receptor. Two types were found cholinoreceptors:
M-receptor,in addition to acetylcholine selectively interacting with muscarin (toxin toxin of the mumor). M - cholinoreceptors are available on smooth muscles and when interacting with acetylcholine causes them to reduce them;
N-receptor,specifically binding with nicotine. H-cholinoreceptors are found in synapses of transverse skeletal muscles.
Specific inhibitor M-cholinoreceptorsis atropine. It is contained in plants handsome and white.
 Atropine has in the structure similar to acetylcholine functional groups and their spatial location, therefore relates to competitive M-cholinoreceptor inhibitors. Given that the binding of acetylcholine with m-cholinoreceptors causes a reduction in smooth muscles, atropine uses as a medicine that relieves their spasm (antispasmodic).So, it is known to use atropine to relax eye muscles when viewing the eye dna, as well as to remove spasms with stomach colic. M-cholinoreceptors are available in Central nervous system (CNS), therefore large doses of atropine can cause an unwanted reaction from the CNS: motor and mental excitation, hallucinations, seizures.
Atropine has in the structure similar to acetylcholine functional groups and their spatial location, therefore relates to competitive M-cholinoreceptor inhibitors. Given that the binding of acetylcholine with m-cholinoreceptors causes a reduction in smooth muscles, atropine uses as a medicine that relieves their spasm (antispasmodic).So, it is known to use atropine to relax eye muscles when viewing the eye dna, as well as to remove spasms with stomach colic. M-cholinoreceptors are available in Central nervous system (CNS), therefore large doses of atropine can cause an unwanted reaction from the CNS: motor and mental excitation, hallucinations, seizures.
Ditylin is a competitive anti-cholinoreceptor agonist inhibiting the function of neuromuscular synapses.
 Nervous muscular synapses of skeletal muscles contain n-cholinoreceptors. Their interaction with acetylcholine leads to muscle contractions. In some surgical operations, and endoscopic studies use drugs that cause relaxation of skeletal muscles (Miorosanta).These include dithiline, which is a structural analogue of acetylcholine. It is joined by n-cholinoreceptors, but, unlike acetylcholine, it is very slowly destroyed by the enzyme - acetylcholinesterase. As a result of a long opening of ion channels and a rack depolarization of the membrane, a nervous impulse is disturbed and muscle relaxation occurs. Initially, these properties were discovered in poison coarara, so such drugs call stripped.
Nervous muscular synapses of skeletal muscles contain n-cholinoreceptors. Their interaction with acetylcholine leads to muscle contractions. In some surgical operations, and endoscopic studies use drugs that cause relaxation of skeletal muscles (Miorosanta).These include dithiline, which is a structural analogue of acetylcholine. It is joined by n-cholinoreceptors, but, unlike acetylcholine, it is very slowly destroyed by the enzyme - acetylcholinesterase. As a result of a long opening of ion channels and a rack depolarization of the membrane, a nervous impulse is disturbed and muscle relaxation occurs. Initially, these properties were discovered in poison coarara, so such drugs call stripped.
Topic 1.3. Denaturation of proteins and the possibility of their spontaneous renatitution
1. Since the native conformation of proteins is maintained due to weak interactions, the change in the composition and properties of the environment of the medium, the effects of chemical reagents and physical factors cause a change in their conformation (the property of conformational lability). The breaking of a large number of links leads to the destruction of the native conformation and denaturation of proteins.
Denaturation of proteins- This is the destruction of their native conformation under the action of denaturing agents caused by a gap of weak bonds that stabilize the spatial structure of the protein. Denaturation is accompanied by the destruction of the unique three-dimensional structure and the active center of the protein and the loss of its biological activity (Fig. 1.16).
All denatured one protein molecules acquire a random conformation, which differs from other molecules of the same protein. Amino acid radicals that form an active center are spatially removed from each other, i.e. A specific protein binding center with ligand is destroyed. With denaturation, the primary structure of proteins remains unchanged.
The use of denaturing agents in biological research and medicine.In biochemical studies, before the determination of low molecular weight compounds, proteins are usually removed from the solution at first. For this purpose, trichloroacetic acid (TCH) is most often used. After adding TCH to the solution, denatured proteins fall into the precipitate and are easily removed by filtration (Table 1.1.)
In medicine, denaturing agents are often used to sterilize the medical instrument and material in autoclaves (denaturing agent - high temperature) and as antiseptics (alcohol, phenol, chlorine) for processing contaminated surfaces containing pathogenic microflora.
2. Spontaneous protein renal- Proof of the determination of the primary structure, conformation and the function of proteins. Individual proteins are the products of one gene, which have an identical amino acid sequence and in the cell acquire the same conformation. The fundamental conclusion that in the primary structure of the protein has already laid information about its conformation and functions, it was made on the basis of the ability of some proteins (in particular, ribonuclease and myoglobin) to spontaneous renativation - the restoration of their native conformation after denaturation.
The formation of spatial protein structures is carried out by the method of self-collecting - spontaneous process, in which the polypeptide chain having a unique primary structure seeks to adopt a conformation with the smallest free energy in solution. The ability to reduce proteins that preserve the primary structure after denaturation is described in the experiment with the enzyme ribonuclease.
Ribonuclease is an enzyme that destroys the relationship between individual nucleotides in the RNA molecule. This globular protein has one polypeptide chain, the tertiary structure of which is stabilized by a multitude of weak and four disulfide bonds.
The treatment of ribonuclease urea, destroying hydrogen bonds in the molecule, and a reducing agent that tears disulfide bonds leads to denaturation of the enzyme and loss of its activity.
The removal of denaturing agents of dialysis leads to the restoration of the conformation and the protein function, i.e. to the renatitution. (Fig. 1.17).
 Fig. 1.17. Denaturation and Renitating Ribonuclease
Fig. 1.17. Denaturation and Renitating Ribonuclease
A - native conformation of ribonuclease, in the tertiary structure of which there are four disulfide bonds; B - denatured ribonuclease molecule;
V - renatative ribonuclease molecule with restored structure and function
1. Fill in Table 1.2.
Table 1.2. Classification of amino acids on the polarity of radicals
2. Write a tetrapeptide formula:
ASP - Pro - Fen - Liz
a) highlight the peptide repeating groups forming the peptide core, and the variable groups represented by amino acid radicals;
b) designate N- and C-ends;
c) emphasize peptide bonds;
d) Write another peptide consisting of the same amino acids;
e) Count the number of possible variants of a tetrapeptide with a similar amino acid composition.
3. Explain the role of the primary structure of proteins on the example of a comparative analysis of two similar in structure and evolutionarly close peptide hormones of the neurohypophysis of mammalian - oxytocin and vasopressin (Table 1.3).
Table 1.3. Structure and functions of oxytocin and vasopressin
 For this:
For this:
a) Compare the composition and sequence of amino acids of two peptides;
b) find similarities of the primary structure of two peptides and the similarity of their biological action;
c) Find the differences in the structure of two peptides and the difference between their functions;
d) Take the conclusion about the effect of the primary structure of peptides on their functions.
4. Describe the main stages of the formation of the conformation of globular proteins (secondary, tertiary structure, the concept of the super-standard structure). Specify the types of connections involved in the formation of protein structures. What amino acid radicals can participate in the formation of hydrophobic interactions, ionic, hydrogen bonds.
Give examples.
5. Give the definition of the "conformational lability of proteins", indicate the causes of its existence and value.
6. Expand the meaning of the next phrase: "The functioning of proteins is based on their specific interaction with the ligand," using terms and explaining their value: protein conformation, active center, ligand, complementarity, protein function.
7. At one example, explain what domains are and what is their role in the functioning of proteins.
Tasks for self-control
1. Set match.
Functional group in amino acid radical:
A. Carboxyl group B. Hydroxyl group in Guanidinovaya Group of Tiolny Group D. Aminogroup
2. Choose the right answers.
Amino acids with polar uncharged radicals - this is:
A. Cis B. ASN
B. Gl. Three
3. Choose the right answers.
Amino acid radicals:
A. Provide the specificity of the primary structure B. Participate in the formation of the tertiary structure
B. located on the surface of the protein, affect its solubility of G. Form an active center
D. Participate in the formation of peptide ties
4. Choose the right answers.
Hydrophobic interactions can be formed between amino acid radicals:
A. Tre Lei B. Pro three
B. MET ILE G. Tir Alla D. Shaft Hairdry
5. Choose the right answers.
Ionic ties can be formed between amino acid radicals:
A. GLN ASP B. Apr Liz
B. Liz Mr. GIS ASP D. ASN APR
6. Choose the right answers.
Hydrogen bonds can be formed between amino acid radicals:
A. Serm GLN B. Cis Tre
B. ASP LIZ G. G-ASP D. ASN Tre
7. Set match.
Communication type involved in the formation of a protein structure:
A. Primary Structure B. Secondary Structure
B. Tertiary structure
G. Superventor structure D. Conformation.
1. Hydrogen bonds between the atoms of the peptide island
2. Weak bonds between the functional groups of amino acid radicals
3. Relations between α-amino and α-carboxyl groups of amino acids
8. Choose the right answers. Tripsin:
A. Proteindic enzyme B. contains two domains
B. Hydrolylizes Starch
The active center is located between the domains. D. consists of two polypeptide chains.
9. Choose the right answers. Atropine:
A. Neurotiator
B. Structural analogue of acetylcholine
B. interacts with n-cholinoreceptors
G. Strengthens Nervous Pulse through Holinergic Sinapses
D. Competitive M-Holnoreceptor Inhibitor
10. Choose the right statements. In proteins:
A. Primary structure contains information about the structure of its active center
B. The active center is formed at the level of the primary structure
B. Conformation is rigidly fixed by covalent bonds
G. Active Center can interact with a group of similar ligands
due to the conformational lability of proteins D. change ambientmay affect the affinity of active
center to Liganda
1. 1-B, 2-g, 3-b.
3. A, B, B, G.
7. 1-b, 2-d, 3-a.
8. A, B, B, G.
Major terms and concepts
1. Protein, polypeptide, amino acids
2. Primary, secondary, tertiary protein structure
3. Conformation, native protein conformation
4. Covalent and weak bonds in protein
5. Conformational lability
6. Active Squirrel Center
7. Ligands
8. Folding Belkov
9. Structural Analogs of Ligands
10. Domain proteins
11. Simple and complex proteins
12. Denaturation of protein, denaturing agents
13. Renitization of proteins
Solve the tasks
"The structural organization of proteins and the foundations of their functioning"
1. The main function of the protein is hemoglobin A (NVA) - the transport of oxygen to the tissues. In the population of people, multiple forms of this protein with changed properties and a function are known - the so-called abnormal hemoglobins. For example, it has been established that hemoglobin S, detected in the erythrocytes of patients with sickle-cell anemia (HBS), has low solubility under conditions of low partial oxygen pressure (as it takes place in venous blood). This leads to the formation of the aggregates of this protein. The protein loses its function, falls into a precipitate, and the erythrocytes acquire the wrong shape (some of them form the shape of the sickle) and faster than the usual destroyed in the spleen. As a result, crespovid cell anemia develops.
The only difference in the primary structure of the NVA and detected in the N-terminal section of the β-chain of hemoglobin. Compare N-terminal sections of the β-chain and show how the changes in the primary protein structure affect its properties and functions.
 For this:
For this:
a) Write the formulas for amino acids, according to which the NVA differ and compare the properties of these amino acids (polarity, charge).
b) Take the conclusion about the reason for the decrease in solubility and disorder of oxygen transport in tissue.
2. The figure shows the scheme of the structure of a protein having a binding center with a ligand (active center). Explain why the protein has selectivity in choosing a ligand. For this:
a) remember what is the active center of the protein, and consider the structure of the active center of the protein presented in the figure;
b) write amino acid radical formulas that are part of the active center;
c) Draw a ligand that could specifically interact with the active center of the protein. Specify on it functional groups that can form connections with amino acid radicals that are part of the active center;
d) specify the types of links arising between the ligand and the radicals of the amino acids of the active center;
e) Explain what the specificity of the interaction of protein with a ligand is based.
 3.
The figure shows the active center of the protein and several ligands.
3.
The figure shows the active center of the protein and several ligands.
Determine which of the ligands with the greatest probability will interact with the active protein center and why.
 What types of links arise in the process of the formation of a protein-ligand complex "?
What types of links arise in the process of the formation of a protein-ligand complex "?
4. The structural analogs of natural ligands of proteins can be used as drugs to change the activity of proteins.
Acetylcholine is a mediator of excitation transmission in neuromuscular synapses. In the interaction of acetylcholine with proteins - the receptors of the postsynaptic membrane of skeletal muscles, the opening of ion channels and muscle contraction occurs. Ditylin is a medicine used in some operations to relax muscles, as it disrupts the transmission of the nervous pulse through neuromuscular synapses. Explain the mechanism of action of dithiline as a mioryexing drug. For this:
a) Write the formulas of acetylcholine and dithiline and compare their structures;
b) Describe the mechanism of relaxing dithiline action.
5. In some diseases, the patient increases the body temperature, which is considered as a protective reaction of the body. However, high temperatures are detrimental to organism proteins. Explain why at temperatures above 40 ° C, the protein function is disturbed and the threat arises for the human life. To do this, remember:
1) the structure of proteins and communication, holding its structure in native conformation;
2) How is the structure and function of proteins change when the temperature is raised?;
3) What is homeostasis and why it is important to maintain human health.
Modular unit 2 oligomeric proteins as a target of regulatory influences. Structural and functional variety of proteins. Methods of separation and cleaning of proteins
The objectives of the study are able to:
1. Use knowledge about the features of the structure and functions of oligomeric proteins to understand the adaptive mechanisms for regulating their functions.
2. Explain the role of chapers in the synthesis and maintaining the conformation of proteins in cell conditions.
3. Explain the diversity of manifestation of the diversity of structures and functions synthesizing in the body of proteins.
4. Analyze the connection of the structure of proteins with their function on the examples of comparing related hemoproteins - Mioglobin and hemoglobin, as well as representatives of the five classes of proteins of the immunoglobulin family.
5. Apply knowledge about the peculiarities of the physicochemical properties of proteins to select the methods of cleaning them from other proteins and impurities.
6. Interpret the results of quantitative and quality composition Plasma proteins for confirmation or clarification of a clinical diagnosis.
Know:
1. Features of the structure of oligomeric proteins and adaptive mechanisms for regulating their functions on the example of hemoglobin.
2. The structure and functions of the chaperons and their value to maintain the native conformation of proteins in cell conditions.
3. The principles of combining proteins in the family to similarize their conformation and functions on the example of immunoglobulins.
4. Methods of separation of proteins based on the characteristics of their physicochemical properties.
5. Blood plasma electrophoresis as a method for assessing the qualitative and quantitative composition of proteins.
Topic 1.4. Features of the structure and functioning of oligomeric proteins on the example of hemoglobin
1. Many proteins have several polypeptide chains in their composition. Such proteins are called oligomericand individual chains - protesters.Protteers in oligomeric proteins are connected by a plurality of weak non-virulent bonds (hydrophobic, ionic, hydrogen). Interaction
protomers are carried out thanks complementaritytheir contacting surfaces.
The number of protéers in oligomeric proteins can vary greatly: hemoglobin contains 4 protsometer, the aspartaminotransferase enzyme is 12 protsometers, and 2120 protéers connected by non-member connections are included in the tobacco mosaic virus protein. Consequently, oligomeric proteins may have a very large molecular weight.
The interaction of one protéer with others can be viewed as a special case of interaction between protein with a ligand, since each fragrance serves as a ligand for other protéers. The amount and method of connecting protometer in protein is called quaternary protein structure.
The proteins may include the same or different protiferers, for example, homodimers - proteins containing two identical protéer, and heterodimers - proteins containing two different protéer.
If the proteins include different protéers, then they can form differ in the structure of binding centers with different ligands. When binding ligands with an active center, the function of this protein is manifested. The center, located on another protester, is called altoherectic (other different from active). Binding S. alosteric ligand or effector,it performs a regulatory function (Fig. 1.18). The interaction of the Alosteric Center with the effector causes conformational changes in the structure of the entire oligomeric protein due to its conformational lability. This affects the affinity of the active center to a specific ligand and regulates the function of this protein. The change in the conformation and the functions of all protisters in the interaction of oligomeric protein at least with one ligand is the name of cooperative changes in the conformation. Effectors reinforcing protein function are called activatorsand effectors who depress it function - inhibitors.
Thus, oligomeric proteins, as well as proteins having a domain structure, a property appears new compared to monomeric proteins - the ability to altogetherteric regulation of functions (regulation by attaching to the protein of different ligands). This can be traced by comparing the structures and functions of two close related complex proteins of myoglobin and hemoglobin.
 Fig. 1.18. Dimensic protein structure
Fig. 1.18. Dimensic protein structure
2. The formation of spatial structures and the functioning of myoglobin.
Mioglobin (MV) - protein located in red muscles, the main function of which is the creation of reserves of 2 required with intensive muscular work. MV is a complex protein containing the protein part - the APOMV and a non-chicken part - gem. The primary structure of the APMU determines its compact globular conformation and the structure of the active center to which the non-skilled part of Mioglobin is joined. Oxygen coming from blood into the muscles is binding to Fe + 2 Gema as part of myoglobin. MV is a monomeric protein that has a very high affinity for o 2, therefore, the return of oxygen by myoglobin occurs only with intense muscle work, when the partial pressure O 2 decreases sharply.
Formation of mV conformation.In red muscles on ribosomes, during the broadcast, the synthesis of the primary structure of the MV, represented by a specific sequence of 153 amino acid residues. The secondary structure of the MV contains eight α-spirals, called Latin letters from A to N, between which there are unpiralized sections. The tertiary structure of the MV has the form of a compact globule, in the deepening of which an active center is located between F and E α-helix (Fig. 1.19).
 Fig. 1.19. Mioglobin structure
Fig. 1.19. Mioglobin structure
3. Features of the structure and operation of the active center of the MV.The MV active center is predominantly hydrophobic amino acid radicals, far away from each other in the primary structure (for example, three 3 9 both hair dryers 138) to the active center are joined by poorly soluble in water ligands - gem and about 2. Gem is a specific ligand of the APMU (Fig. 1.20), the basis of which is four pyrrole rings connected by the Metric Bridges; In the center there is an FE + 2 atom, connected to the nitrogen atoms of pyrrolean rings by four coordination bonds. In the active center of the MV, in addition to the hydrophobic amino acid radicals, there are also residues of two amino acids with hydrophilic radicals - GIS E 7.(GIS 64) and GIS F 8.(GIS 93) (Fig. 1.21).
 Fig. 1.20. The structure of the gem - non-discovered Mioglobin and hemoglobin
Fig. 1.20. The structure of the gem - non-discovered Mioglobin and hemoglobin
 Fig. 1.21. GEMA and O 2 location in the active center of apomoglobin and hemoglobin propters
Fig. 1.21. GEMA and O 2 location in the active center of apomoglobin and hemoglobin propters
Gem through an iron atom is covalently associated with GIS F 8. O 2 joins the gland on the other side of the heme plane. GIS E 7 is necessary for the correct orientation of 2 and facilitates the addition of oxygen to Fe + 2 hem
GIS F 8.forms coordination with Fe + 2 and firmly fixes gem in the active center. GIS E 7.we are necessary for proper orientation in the active center of another ligand - O 2 when it interacts with Fe + 2 Gema. Gema micro-operation creates conditions for durable, but reversible binding O 2 with Fe +2 and prevents the water in the hydrophobic active center, which can lead to its oxidation in Fe + 3.
The monomer structure of the MV and its active center determines the high affinity of the protein to 2.
4. The oligomeric structure of the HB and the regulation of the affinity of the HB to 2 ligands. Human hemoglobins- Family of proteins, as well as Mioglobin related to complex proteins (hemoproteins). They have a tetramer structure and contain two α-chains, but differ in the structure of two other polypeptide chains (2α-, 2x chains). The structure of the second polypeptide chain determines the features of the functioning of these forms of HV. About 98% of the hemoglobin of the erythrocytes of an adult is hemoglobin A.(2α-, 2p chains).
During the period of intrauterine development, two main types of hemoglobins are functioning: embryonic NV(2α, 2ε), which is found to early stages fetus development and hemoglobin f (fetal)- (2α, 2γ), which comes to replace the early hemoglobin of the fetus on the sixth month of intrauterine development and only after birth is replaced by NV A.
NV A - protein, related Mioglobin (MV), is contained in the erythrocytes of an adult. The structure of its individual protéers is similar to that of Mioglobin. The secondary and tertiary structure of myoglobin and hemoglobin propters are very similar, despite the fact that only 24 amino acid residues are identical in the primary structure of their polypeptide chains (the secondary structure of hemoglobin protesters, as well as Mioglobin, contains eight α-spirals denoted by Latin letters from A BC and the tertiary structure has the form of a compact globule). But, in contrast to myoglobin, hemoglobin has an oligomeric structure, consists of four polypeptide chains connected by non-covalent bonds (Fig. 1.22).
Each protiber HB is associated with a non-discovered part of the gem and adjacent protesters. The combustion of the protein part of the HV with a gem is similar to that of Mioglobin: in the active center of the protein, the hydrophobic gem hydrophobic parts are surrounded by hydrophobic amino acid radicals with the exception of GIS F 8 and GIS E 7, which are located on both sides of the heme plane and play a similar role in the functioning of the protein and binding it with oxygen (see the structure of myoglobin).
 Fig. 1.22. Oligomeric structure of hemoglobin
Fig. 1.22. Oligomeric structure of hemoglobin
Moreover, GIS E 7.performs important additional rolein the functioning of the HB. Free gem has 25,000 times higher affinity for CO than K O 2. CO in small quantities is formed in the body and, given its high affinity for the G., he could violate the transport of the cells necessary for the life of 2. However, as part of the hemoglobin affinity of the hem to carbon oxide exceeds the affinity of 2 only 200 times due to the presence in the active center of GIS E 7. The residue of this amino acid creates optimal conditions for the binding of the heme with O 2 and weakens the interaction of the heme with CO.
5. The main function of the NV is transport about 2 of the lungs in the fabric.Unlike monomeric myoglobin, having a very high affinity to O 2 and performs the function of oxygen in red muscles, the oligomeric structure of hemoglobin provides:
1) rapid saturation of HV oxygen in the lungs;
2) the ability of HB to give oxygen in tissues at a relatively high partial pressure O 2 (20-40 mm Hg. Art.);
3) the possibility of regulating the affinity of the HB to O 2.
6. Cooperative changes in the conformation of the propters of hemoglobin accelerate the binding O 2 in the lungs and the returns to the tissue. In the lungs, the high partial pressure O 2 contributes to the binding of it with HB in the active center of four protéer (2α and 2β). The active center of each protéer, as well as in myoglobin, is located between two α-spirals (F and E) in the hydrophobic pocket. It contains a non-pecker part - gem attached to the protein part with a plurality of weak hydrophobic interactions and one durable bond between Fe 2 + Gema and GIS F 8 (see Fig. 1.21).
In deoxyhemoglobin, thanks to this connection with GIS F 8, the FE 2 + atom is from the heme plane towards histidine. The binding O 2 with Fe 2 + occurs on the other side of the heme in the GIS E 7 area using a single free coordination. GIS E 7 provides optimal conditions for binding O 2 with the Heme Iron.
The addition of O 2 to the FE +2 one proteter atom causes its movement to the heme plane, and the residue of the histinea associated with it
 Fig. 1.23. Changes in the conformation of the propters of hemoglobin when connected with O 2
Fig. 1.23. Changes in the conformation of the propters of hemoglobin when connected with O 2
This leads to a change in the conformation of all polypeptide chains due to their conformational lability. The change in the conformation of other chains facilitates their interaction with the following molecules about 2.
The fourth molecule O 2 joins hemoglobin 300 times easier than the first (Fig. 1.24).
 Fig. 1.24. Cooperative changes in the conformation of the propters of hemoglobin in its interaction with O 2
Fig. 1.24. Cooperative changes in the conformation of the propters of hemoglobin in its interaction with O 2
In the tissues, each next O 2 molecule is cleaned easier than the previous one, also due to cooperative changes in the conformation of protesters.
7. CO 2 and H +, which are formed during the catabolism of organic substances, reduce the affinity of hemoglobin to o 2 in proportion to their concentration. The energy required for the operation of the cells is produced mainly in mitochondria when oxidizing organic substances using O 2 delivered from light hemoglobin. As a result of the oxidation of organic substances, final products of their decay are formed: CO 2 and K 2 O, the number of which is proportional to the intensity of oxidation processes.
CO 2 diffusion gets out of cells into blood and penetrates erythrocytes, where, under the action of the enzyme carbanthidase turns into coalic acid. This weak acid dissociates to proton and the ion bicarbonate.
N + capable of joining radicals GIS 14 6 in α- and β-chains of hemoglobin, i.e. In areas remote from hem. The protonation of hemoglobin reduces its affinity to O 2, contributes to the cleavage of O 2 on oxyne, the formation of deoxynev and increases the flow of oxygen in tissue in proportion to the number of protons formed (Fig. 1.25).
An increase in the amount of freed oxygen, depending on the increase in the concentration of H + in erythrocytes, is called the effect of boron (named Danish physiologist Christian Bohr, who first discovered this effect).
In the lungs, the high partial pressure of oxygen contributes to its binding to disoxyn, which reduces the affinity of the protein to H +. Released protons under the action of carbanthidases interact with bicarbonates with the formation of CO 2 and H 2

 Fig. 1.25. The dependence of the affinity of the HB to O 2 on the concentration of CO 2 and protons (Bora effect):
Fig. 1.25. The dependence of the affinity of the HB to O 2 on the concentration of CO 2 and protons (Bora effect):
BUT- influence of the concentration of CO 2 and H + on the release of 2 of the complex with HB (Bohr Effect); B.- oxygenation of deoxyhemoglobin in the lungs, education and allocation of CO 2.
The resulting CO 2 enters the alveolar space and removed with the exhaled air. Thus, the amount of hemoglobin oxygen released in tissues is regulated by catabolic products of organic substances: the more intense decay of substances, for example, during physical exertion, the higher the concentration of CO 2 and H + and the greater the oxygen is obtained by the tissue as a result of the reduction of the affinity of HB to O 2.
8. Alosteric regulation of HB affinity to 2 ligand - 2,3-bisphosphoglycerat.In red blood cells from the glucose oxidation product - 1,3-bisphosphoglycerat is synthesized by alto-cell league hemoglobin - 2,3-bisphosphoglycerat (2,3-BFG). Under normal conditions, the concentration of 2.3-BFG is high and comparable to the HV concentration. 2,3-BFG has a strong negative charge -5.
 Bisphosphoglycerat in tissue capillaries, binding to deoxyhemoglobin, increases the yield of oxygen in tissue, reducing the affinity of the HB to O 2.
Bisphosphoglycerat in tissue capillaries, binding to deoxyhemoglobin, increases the yield of oxygen in tissue, reducing the affinity of the HB to O 2.
In the center of the tetramer hemoglobin molecule is the cavity. It forms the amino acid residues of all four protéers (see Fig. 1.22). In the capillaries of tissues, the protonation of the HB (boron effect) leads to a rupture of communication between the heme and 2 gland. In molecule
deoxyhemoglobin compared to oxymemoglobin ion tiesconnecting propters, as a result of which the size of the central cavity compared to the oxymemoglobin increases. The central cavity is a 2,3-BFG joining place to hemoglobin. Due to the differences in the size of the central cavity, 2,3-BFG can only be joined by deoxyhemoglobin.
2,3-BFG interacts with hemoglobin in a plot removed from the active protein centers and belongs to alosteric(regulatory) ligands, and the central cavity of the HB is alosteric center.2.3-BFG has a strong negative charge and interacts with five positively charged groups of two β-chains of HB: N-terminal α-amino group shaft and Liz radicals 82 GIS 143 (Fig. 1.26).
 Fig. 1.26. BFG in the central cavity of deoxyhemoglobin
Fig. 1.26. BFG in the central cavity of deoxyhemoglobin
BFG binds to three positively charged groups in each β-chain.
In the capillaries of tissues, the deoxyhemoglobin is interacting with 2,3-BFG and between positively charged β-chain radicals and a negatively charged ligand formed ion bonds that change the conformation of the protein and reduce the affinity of the HB to O 2. Reducing the affinity of the HB to O 2 contributes to a more effective output of 2 in tissue.
In the lungs at high partial pressure, oxygen interacts with HB, joining the glame gland; In this case, the conformation of the protein changes, the central cavity decreases and 2,3-BFG from the alto-solid center is displaced.
Thus, oligomeric proteins have new properties compared to monomeric proteins. Attaching ligands in areas,
spatially removed from each other (altowork), can cause conformational changes in the entire protein molecule. Due to the interaction with the regulatory ligands, the conformation is changed and the adaptation of the function of the protein molecule to environmental changes.
Topic 1.5. Maintaining the native conformation of proteins in cell conditions
In the cells in the process of the synthesis of polypeptide chains, their vehicles through the membranes into the corresponding cells, during the process of folding (the formation of native conformation) and during the assembly of oligomeric proteins, and in the period of their functioning in the structure of proteins, intermediate, prone to aggregation, unstable conformations arise. Hydrophobic radicals, in native conformation, usually hidden inside the protein molecule, in the unstable conformation are on the surface and strive for combining with the same poorly soluble groups in water with groups of other proteins. In the cells of all known organisms, special proteins were found, which provide optimal fildising cell proteins, stabilize their native conformation during operation and, which is especially important, support the structure and functions of intracellular proteins with a violation of homeostasis. These proteins got a name "Chaperons",that translated from French means "nanny".
1. Molecular chaperons and their role in preventing protein denaturation.
Shaperons (W) are classified by weight of subunits. High molecular weight shaperons have a mass from 60 to 110 kD. Among them are the most studied three classes: W-60, WC-70 and W-90. Each class includes a family of related proteins. Thus, the composition of W-70 includes proteins with a molecular weight from 66 to 78 kD. Low molecular weight shaperons have a molecular weight from 40 to 15 kD.
Among Chaperons are distinguished constituticalproteins, high basal synthesis of which does not depend on stressful effects on the cells of the body, and induciblethe synthesis of which under normal conditions is weak, but increases sharply during stressful effects. Inductile shaperons are also called "proteins of heat shock", since they were first discovered in cells undergoing high temperatures. In cells due to the high concentration of proteins, spontaneous renatitution of partially denatured proteins is hampered. The WC-70 can prevent the resulting denaturation process and promote the restoration of the native conformation of proteins. Molecular Chaperons-70- High-circuit class of proteins located in all cells of the cell: cytoplasm, core, endoplasmic reticulum, mitochondria. At the carboxyl end of the only polypeptide circuit of W-70, there is a plot that is a groove capable of interacting with peptides
from 7 to 9 amino acid residues enriched with hydrophobic radicals. Such areas in globular proteins occur around every 16 amino acids. The WC-70 is capable of protecting proteins from temperature inactivation and restore the conformation and activity of partially denatured proteins.
2. The role of chapers in Folding proteins.In the synthesis of proteins on the ribosome, the N-terminal region of the polypeptide is synthesized before the C-terminal. To form a native conformation, a complete amino acid sequence of protein is necessary. In the process of the synthesis of the Sperlons-70 proteins, due to the structure of their active center, the areas of the polypeptide prone to aggregation, enriched with hydrophobic radicals of amino acids prior to the completion of the synthesis (Fig. 1.27, a).
 Fig. 1.27. Participation of Chaperons in Folding Proteins
Fig. 1.27. Participation of Chaperons in Folding Proteins
A - the participation of shaperone-70 in preventing hydrophobic interactions between the sections of the synthesized polypeptide; B - Formation of native protein conformation in a chaperon complex
Many high molecular weight proteins having a complex conformation, such as a domain structure, produce folding in a special space formed by W-60. Sh-60function in the form of an oligomeric complex consisting of 14 subunits. They form two hollow rings, each of which consists of seven subunits, these rings are connected to each other. Each subunit W-60 consists of three domains: apical (top), enriched with hydrophobic radicals facing the rings, intermediate and equatorial cavity (Fig. 1.28).
 Fig. 1.28. The structure of the shaperonin complex consisting of 14 W-60
Fig. 1.28. The structure of the shaperonin complex consisting of 14 W-60
A - side view; B - top view
Synthesized proteins that have elements on the surface characteristic of unfolded molecules, in particular hydrophobic radicals, fall into the cavity of the shaperone rings. In a specific environment of these cavities, there is a bust of possible conformations, until the only one, the energy is most beneficial (Fig. 1.27, b). The formation of conformations and the release of protein is accompanied by hydrolysis of ATP in the equatorial region. Usually such a shaperone-dependent folding requires the costs of a significant amount of energy.
In addition to participating in the formation of a three-dimensional structure of proteins and the renatitution of partially denatured proteins, the shaperons are also necessary for the flow of such fundamental processes as the assembly of oligomeric proteins, recognition and transport in the lysosomes of denatured proteins, protein transport through membranes, participation in the regulation of protein complexes.
Topic 1.6. Variety of proteins. Family of proteins on the example of immunoglobulins
1. Proteins play a crucial role in the vital activity of individual cells and all the multicellular organism, and their functions are surprisingly diverse. This is determined by the peculiarities of the primary structure and conformations of proteins, the uniqueness of the structure of the active center and the ability to associate specific ligands.
Only a very small part of all possible variants of peptide chains can take a stable spatial structure; most
of these, it can take many conformations with about the same Gibbs energy, but with various properties. The primary structure of most famous proteins selected biological evolution, ensures the exceptional stability of one of the conformations, which determines the features of the functioning of this protein.
2. Family proteins.Within a single biological type of replacement of amino acid residues can lead to the occurrence of different proteins that perform related functions and having homologous sequences Amino acids. Such related proteins have strikingly similar conformation: the number and interposition of α-helix and (or) β-structures, most of the turns and bends of polypeptide chains are similar or identical. Proteins with homologous sections of the polypeptide chain, similar conformation and related functions are isolated in the protein family. Examples of proteins families: serine proteinases, family of immunoglobulins, Mioglobin family.
Serine proteinases- Family of proteins that perform the function of proteolytic enzymes. These include digestive enzymes - chymotrypsin, trypsin, elastase and many coagulation factors. These proteins have in 40% of the provisions of identical amino acids and a very close conformation (Fig. 1.29).
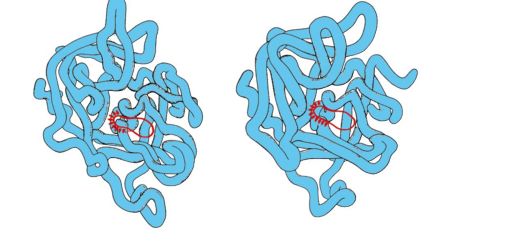
Fig. 1.29. Spatial structures of elastase (a) and chymotrypsin (b)
Some amino acid substitutions led to a change in the substrate specificity of these proteins and the emergence of a functional manifold within the family.
3. Family of immunoglobulins.In the work of the immune system, proteins of superfamily immunoglobulinov play a huge role, which includes three families of proteins:
Antibodies (immunoglobulins);
T-lymphocyte receptors;
The proteins of the main histocompatibility complex - MAJOR HISTOCOMPATILITY COMPLEX).
All these proteins have a domain structure, consist of homologous immunopod-like domains and carry out similar functions: interact with alien structures, or dissolved in blood, lymph or intercellular fluid (antibodies), or on the surface of cells (own or alien).
4. Antibodies- Specific proteins produced by in lymphocytes in response to entering the body of a foreign structure called antigen.
The features of the structure of antibodies
The simplest antibody molecules consist of four polypeptide chains: two identical lungs - L containing about 220 amino acids, and two identical heavy-H, consisting of 440-700 amino acids. All four chains in the antibody molecule are connected by a plurality of non-covalent bonds and four disulfide bonds (Fig. 1.30).
The light chains of the antibody consist of two domains: variable (VL), which is located in the N-terminal region of the polypeptide chain, and constant (CL), located on the end. Heavy chains usually have four domains: one variable (VH), located on the N-end, and three constant (CH1, CH2, SNZ) (see Fig. 1.30). Each immunoglobulin domain has a β-folded superstructure, in which two cysteine \u200b\u200bresidue is connected by disulfide bond.
Between the two constant domains of CH1 and CH2 there is a plot containing a large number of proline residues that impede the formation of the secondary structure and the interaction of neighboring H-chains on this segment. This hinge area gives the molecule of the antibody flexibility. There are two identical antigen-binding plots between variable domains of heavy and light chains (active centers for antigen binding), so such antibodies are often called bivalent.In the binding of the antigen with an antibody, not the entire amino acid sequence of variable sections of both chains of both chains is involved, and only 20-30 amino acids located in the hypervariable regions of each chain. It is these areas that determine the unique ability of each type of antibody to interact with the corresponding complementary antigen.
Antibodies are one of the body protection lines against the imaginary alien organisms. Their functioning can be divided into two stages: the first stage is the recognition and binding of the antigen on the surface of alien organisms, which is possible due to the presence of antigen-binding antibodies in the structure; The second stage is the initiation of the process of inactivation and destruction of the antigen. The specificity of the second stage depends on the class of antibodies. There are five classes of heavy chains that differ from each other on the structure of constant domains: α, δ, ε, γ and μ, in accordance with which the five classes of immunoglobulins distinguish: A, D, E, G, and M.
Features of the structure of heavy chains are attached to hinged areas and the C-terminal areas of heavy chains characteristic of each class conformation. After binding an antigen with an antibody, conformational changes in constant domains define the removal path of the antigen.
 Fig. 1. 30. IGG domain structure
Fig. 1. 30. IGG domain structure
Immunoglobulins M.
Immunoglobulins M have two forms.
Monomeric form- 1st class of antibodies produced by developing in-lymphocyte. Subsequently, many B cells switched to the production of other classes of antibodies, but with the same antigen binding site. IgM is embedded in the membrane and performs the role of an antigensnial receptor. Embedding IgM in the cell membrane is possible due to the presence of hydrophobic amino acid residues in the tail portion.
Secretor form Igm.contains five monomer subunits associated with each other disulfide bonds and an additional polypeptide J-chain (Fig. 1.31). Heavy monomer chains of this form do not contain a hydrophobic tail. The pentair has 10 binding centers with an antigen and is therefore effective in recognition and removal of the first antigen in the body. The secretory form IgM is the main class of antibodies secreted into the blood during a primary immune response. The IGM binding with an antigen changes the IGM conformation and induces binding it to the first protein component of the complement system (the complement system is a set of proteins involved in the destruction of the antigen) and the activation of this system. If the antigen is located on the surface of the microorganism, the complement system causes a violation of integrity cell membrane and the death of a bacterial cell.
Immunoglobulins G.
In quantitatively, this class of immunoglobulins prevails in the blood (75% of all Ig). IgG - monomers, the main class of antibodies secreted into the blood during a secondary immune response. After the interaction of IgG with surface antigens of microorganisms, the antigen-antibody complex is able to bind and activate the proteins of the complement system or can interact with specific macrophage and neutrophil receptors. Interaction with phagocytes leads
 Fig. 1.31. Structure of the Secretory Form IGM
Fig. 1.31. Structure of the Secretory Form IGM
to the absorption of antigen-antibody complexes and their destruction in the cells of the cells. IgG is the only class of antibodies that can penetrate the placental barrier and provide intrauterine protection of the fetus from infections.
Immunoglobulins A.
The main class of antibodies present in secrets (milk, saliva, the secrets of the respiratory tract and intestinal tract). IgA secrets mainly in a dimer form, where monomers are associated with each other through an additional J-chain (Fig. 1.32).
Iga does not interact with the complement system and phagocytifying cells, but, binding to microorganisms, antibodies prevent them with accession to epithelial cells and penetration into the body.
Immunoglobulins E.
Immunoglobulins E are represented by monomers in which heavy ε-chains contain, as well as μ-chains of immunoglobulin m, one variable and four constant domains. IgE after secretion bind to their
 Fig. 1.32. The structure of iga.
Fig. 1.32. The structure of iga.
C-terminal sections with appropriate receptors on the surface of obese cells and basophils. As a result, they become receptors for antigens on the surface of these cells (Fig. 1.33).
 Fig. 1.33. IgE interaction with antigen on the surface of the fat cell
Fig. 1.33. IgE interaction with antigen on the surface of the fat cell
After the antigen attachment is attached to the corresponding antigen-binding IgE sites, the cells receive a signal to the secretion of biologically active substances (histamine, serotonin), which are largely responsible for the development of the inflammatory response and for the manifestation of such allergic reactions such as asthma, urticaria, hay fever.
Immunoglobulins D.
Immunoglobulins D are detected in serum in a very small quantity, they are monomers. In heavy δ-chains there are one variable and three constant domains. IgD performs the role of in-lymphocyte receptors, other functions are still unknown. The interaction of specific antigens with receptors on the surface of B-lymphocytes (IGD) leads to the transmission of these signals into the cell and the inclusion of mechanisms that ensure the reproduction of this clone of lymphocytes.
Topic 1.7. Physico-chemical properties of proteins and methods of their separation
1. Individual proteins differ in physicochemical properties:
Molecules;
Molecular weight;
Total charge, the value of which depends on the ratio of anionic and cationic groups of amino acids;
The ratio of polar and non-polar radicals of amino acids on the surface of molecules;
The degree of resistance to the effects of various denaturing agents.
2. Solubility proteins dependsthe properties of the proteins listed above, as well as on the composition of the medium in which the protein is dissolved (pH values, salt composition, temperature, the presence of other organic substances capable of interacting with the protein). The magnitude of the charge of protein molecules is one of the factors affecting their solubility. If the charge loss in the isoelectric point, the proteins are easier to be easier and falling into a precipitate. This is especially characteristic of denatured proteins, in which hydrophobic amino acid radicals are on the surface.
On the surface of the protein molecule, there are both positive and negatively charged amino acid radicals. The number of these groups, and consequently, the total charge of proteins depend on the pH of the medium, i.e. The ratio of the concentration of H + - and it is -groups. In an acidic environmentan increase in the concentration of H + leads to the suppression of the dissociation of carboxyl groups -SOO - + H +\u003e - coxy and lowering the negative charge of proteins. In an alkaline medium, the binding of an excess of it - protons formed during the dissociation of amino groups -NH 3 + + it - - NH 2 + H 2 o with the formation of water leads to a decrease in the positive charge of proteins. The pH value in which the protein has a total zero charge, called isoelectric point (IET).In IET, the number of positive and negatively charged groups is equally, i.e. The protein is in isoelectric condition.
3. Separation of individual proteins.Features of the structure and functioning of the body depend on the set of proteins that synthesize in it. The study of the structure and properties of proteins is impossible without their release from the cell and purification from other proteins and organic molecules. Stages of selection and purification of individual proteins:
Destruction of cellsthe tissue is studied and obtaining homogenate.
Separation of homogenate on fractionscentrifugation, obtaining a nuclear, mitochondrial, cytosolic or other fraction containing the desired protein.
Electoral thermal denaturation- short-term heating of protein solution, at which you can remove part of the deuted protein impurities (if protein relative to thermostable).
Planting.Various proteins fall into precipitate at different salt concentrations in solution. Gradually, increasing the concentration of salt, one can obtain a number of individual fractions with the predominant content of the protein released in one of them. Most often for fractionation of proteins, ammonium sulphate is used. Proteins with the smallest solubility fall into precipitate at small saline concentrations.
Gel filtration- Method of sieving molecules through the swollen Granules of Sephadex (three-dimensional polysaccharide decastric chains, having pores). The speed of passing proteins through a column filled with Sephadex will depend on their molecular weight: the less weight of the protein molecules, the easier they penetrate the inside of the granules and longer are delayed there, the greater the mass, the faster they elute from the column.
Ultracentrifugation- The method that consists in the fact that proteins in the centrifuge tube are placed in the Ultracentrifuge rotor. When rotating the rotor, the rate of sedimentation of proteins is proportional to their molecular weight: fractions of heavier proteins are closer to the bottom of the test tube, lighter - closer to the surface.
Electrophoresis- The method based on the differences in the speed of movement of proteins in the electric field. This magnitude is proportional to the charge of proteins. Electrophoresis proteins are carried out on paper (in this case, the speed of movement of proteins is proportional only to their charge) or in a polyacrylamide gel with a certain amount of pores (the speed of movement of proteins is proportional to their charge and molecular weight).
Ion exchange chromatography- fractionation method based on binding ionized groups of proteins with oppositely charged groups of ion exchange resins (insoluble polymeric materials). Protein binding strength with a resin is proportional to the package of protein. Proteins adsorbed on an ion-exchange polymer can be washed with NaCl solutions with increasing concentrations; The less protein charge, the smaller NaCl concentration will be required to wash off the protein associated with the ionic groups of the resin.
Affinity chromatography- The most specific method of isolating individual proteins. The inert polymer is covalently joined by the ligand of any protein. When the squad solution is passed through the column with a polymer due to complementary binding of a protein of a protein with a ligand on the column, only a specific protein-specific protein is adsorbed.
Dialysis- The method used to remove low molecular weight compounds from a solution of selected protein. The method is based on the inability of proteins to pass through the semi-permeable membrane, in contrast to low molecular weight substances. It is used to purify proteins from low molecular weight impurities, such as salts after planting.
Tasks for extracurricular work
1. Fill in Table. 1.4.
Table 1.4. Comparative analysis The structures and functions of related proteins - myoglobin and hemoglobin
a) Recall the structure of the active center of the MV and NC. What role does the hydrophobic amino acid radicals play in the formation of active centers of these proteins? Describe the structure of the active center of the MV and HY and the mechanisms of attachment to it ligands. What role is the remnants of GIS F 8 and GIS E 7 in the functioning of the active center of MV Inv?
b) What new properties are closely related oligomeric protein in comparison with monomeric myoglobin - hemoglobin? Explain the role of cooperative changes in the conformation of the protesters in the hemoglobin molecule, the effect of concentrations of CO 2 and protons to the affinity of hemoglobin to oxygen, as well as the role of 2,3-BFG in the alto-using the function of the NC function.
2. Give the characteristic of molecular chapers, paying attention to the connection of their structure with the function.
3. What proteins are united in families? Using the example of the family of immunoglobulins, determine the similarities of the structure and related functions of proteins of this family.
4. Often, purified individual proteins are required for biochemical and medical purposes. Explain that the physicochemical properties of proteins are based on the methods of their separation and cleaning.
Tasks for self-control
1. Choose the right answers.
Gemoglobin functions:
A. Transportation of 2 of the lungs in tissue B. Transport N + from fabrics in lungs
B. Maintaining the constancy of blood pH of the city of Transport Co 2 of the lungs in the fabric
D. Transport Co 2 of the fabrics in the lungs
2. Choose the right answers. Ligandα -Protomer NV is:A. Gem.
B. Oxygen
B. 2,3-BFG
D. β-proper
3. Choose the right answers.
Hemoglobin in contrast to Mioglobin:
A. Has a Quaternary structure
B. Secondary structure is represented by α-spirals
B. Refers to complex proteins.
G. interacts with Alosteric ligand D. Covalently connected with the hem
4. Choose the right answers.
The affinity of the HB to 2 decreases:
A. When attaching one molecule about 2 B. When cleaving one molecule O 2
B. When interacting with 2,3-BFG
G. When connected to the protometer H + D. With a decrease in concentration of 2.3-BFG
5. Set match.
For types of HB, it is characteristic:
A. Deoxy form forms Fibrillar units B. contains in the composition of two α- and two δ-chains
B. The prevailing form of HB in the erythrocytes of an adult man in the active center contains gem with Fe + 3
D. contains two α- and two γ-chains 1. NVA 2.
6. Set match.
Ligands NV:
A. Binds to HB in the Al Motor Center
B. has a very high affinity for the active center of HB
B. By connecting, increases the affinity of the HB to O 2 oxidizes Fe + 2 in Fe + 3
D. forms covalent communication with GISF8.
7. Choose the right answers.
Chaperons:
A. Proteins present in all cells of the cell
B. Synthesis is enhanced in stressful effects.
B. Participate in hydrolysis of denatured proteins
G. are involved in maintaining native protein conformation
D. Create organelles in which the conformation of proteins is formed
8. Install the match. Immunoglobulins:
A. The secretory form has a pentar form
B. Class Ig penetrating a placental barrier
B. Ig - Fat cell receptor
G. Main Class IG present in the secrets of epithelial cells. D. B-lymphocyte receptor, the activation of which ensures the reproduction of cells
9. Choose the right answers.
Immunoglobulins E:
A. Macrophages B. are produced by heavy ε-chains.
B. Embed into the T-lymphocyte membrane
G. Make the role of membrane antigens receptors on fat cells and basophilas
D. Responsible for the manifestation of allergic reactions
10. Choose the right answers.
The method of separation of proteins is based on differences in their molecular weight:
A. Gel filtration
B. ultracentrifugation
B. Electrophoresis in polyacrylamide gel ion exchange chromatography
D. affinity chromatography
11. Choose the correct answer.
The method of separation of proteins is based on differences in their solubility in water:
A. Gel filtration B. planting
B. ion exchange chromatography of affine chromatography
D. Electrophoresis in polyacrylamide gel
Standards of answers to "Jobs for self-control"
1. A, B, B, D
2. A, B, B, D
5. 1-B, 2-A, 3-g
6. 1-B, 2-B, 3-A
7. A, B, G, D
8. 1-g; 2-b, 3-in
Major terms and concepts
1. Oligomeric squirrels, proper, quaternary protein structure
2. Cooperative changes in the conformation of protesters
3. Boron effect
4. Alosteric regulation of the functions of proteins, Alosteric Center and Alosteric Effector
5. Molecular chaperons, heat shock proteins
6. Family proteins (serine proteases, immunoglobulins)
7. IgM-, G-, E-, A-bond structure with function
8. Summary protein charge, protein isoelectric point
9. Electrophoresis
10. Singing
11. Gel filtration
12. ion exchange chromatography
13. Ultracentrifugation
14. Affine chromatography
15. Electrophoresis of blood plasma proteins
Tasks for auditing work
1. Compare the dependences of hemoglobin saturation degrees (HB) and myoglobin (MB) oxygen from its partial pressure in the tissues
 Fig. 1.34. The dependence of the saturation of MV andNyoxygen from its partial pressure
Fig. 1.34. The dependence of the saturation of MV andNyoxygen from its partial pressure
Please note that the shape of protein saturation curves of oxygen is different: for myoglobin - hyperbole, for hemoglobin - a sigmoid form.
1. Compare the values \u200b\u200bof the partial oxygen pressure, in which MB and HV are saturated with 2 by 50%. Which of these proteins is characterized by a higher affinity for 2?
2. What features of the MV structure determine its high affinity for 2?
3. What features of the structure of the NV allow him to give 2 in the capillaries of resting tissues (with relatively high partial pressure of 2) and dramatically increase this return in working muscles? What property of oligomeric proteins provides this effect?
4. Calculate how quantity of 2 (in%) gives an oxygenated hemoglobin resting and working muscle?
5. Make conclusions about the connection of the protein structure with its function.
2. The amount of oxygen released by hemoglobin in capillaries depends on the intensity of catabolism processes in tissues (boron effect). How is the changes in metabolism in tissues regulate the affinity of Ny to O 2? Effect of CO 2 and H + on the affinity of Ny to 2
 1. Describe the boron effect.
1. Describe the boron effect.
2. In which direction the process presented in the scheme proceeds:
a) in the lung capillaries;
b) in climb capillaries?
3. What is the physiological meaning of the boron effect?
4. Why is the interaction of NV with H + in areas remote from the hem, changes the affinity of the protein to 2?
3. The affinity of the NBE to O 2 depends on the concentration of its ligand - 2,3-biphosfo-glycerate, which is the alto-smoking regulator of the affinity of HV to O 2. Why is the interaction of ligand in a plot remote from the active center affect the protein function? How does the 2,3-BFG regulate the affinity of Ny to 2? To solve the task, answer the following questions:
1. Where and from which 2.3-bifosphoglycerat (2,3-BFG) is synthesized? Write it to the formula, specify the charge of this molecule.
2. With which form of hemoglobin (Oxy or Deoxo) interacts the BFG and why? In which section of the HB molecule is interaction?
3. In which direction the process presented in the scheme proceeds
a) in cloth capillaries;
b) in the lung capillaries?
4. Where should be a higher concentration of the complex
HB-2,3-BFG:
a) in the capillaries of muscles in the state of rest
b) in capillaries of working muscles (provided the same concentration of BFG in red blood cells)?
5. How will the affinity of the HV be changed to oxygen when adapting a person to the conditions of the highlands, if the concentration of the BFG in the erythrocytes increases? What is the physiological significance of this phenomenon?
4. The destruction of 2.3-BFG during the storage of canned blood disrupts the functions of the NV. How the affinity of HV is changed to O 2 in canned blood, if the concentration of 2,3-BFG in red blood cells can decrease from 8 to 0.5 mmol / l. Is it possible to overflow such blood to seriously ill patients if the concentration of 2,3-BFG is restored not earlier than in three days? Is it possible to add 2.3-BFG to the blood to restore the functions of erythrocytes?
5. Recall the structure of the simplest immunoglobulin molecules. What role do immunoglobulins play in the work of the immune system? Why is IG are often called bivalent? How is IG structure related to their function? (Describe an example of any class of immunoglobulins.)
Physico-chemical properties of proteins and methods for their separation.
6. How does the protein shade affect its solubility?
a) Determine the total peptide charge at pH 7
Ala-Glu-Tre-ASP-Liz Cis
b) How to change the charge of this peptide at pH\u003e 7, pH<7, рН <<7?
c) what is the isoelectric point of the protein (IET) and in which environment lies
IET of this peptide?
d) with what the meaning of the pH will be observed the smallest solubility of this peptide.
7. Why is acidic milk in contrast to fresh at boiling "folded" (i.e. milk protein Casein falls into the sediment)? In the fresh milk, casein molecules have a negative charge.
8. For the separation of individual proteins, the gelfiltration method is used. A mixture containing proteins A, B, C with molecular masses equal to 160,000, 80,000 and 60,000, respectively, was analyzed by gel filtration (Fig. 1.35). The pellets of the swollen gel permeable for proteins with a molecular weight of less than 70,000. What principle is based on this separation method? Which of the graphs correctly reflects the results of fractionation? Specify the order of the exit of proteins A, B and C from the column.
 Fig. 1.35. Using the gel filtering method for protein separation
Fig. 1.35. Using the gel filtering method for protein separation
9. In fig. 1.36, and shows the electrophoresis diagram on paper protein proteins of a healthy person. The relative amounts of protein fractions obtained using this method are: albumin 54-58%, α 1 -Globulins 6-7%, α 2 -Globulins 8-9%, β-globulines 13%, γ-globulines 11-12% .
 Fig. 1.36 Electrophoresis on paper plasma proteins of a healthy person (s) and patient (b)
Fig. 1.36 Electrophoresis on paper plasma proteins of a healthy person (s) and patient (b)
I - γ-globulins; II - β-globulines; III -α 2-Globulin; IV -α 2-Globulin; V - Albumin
Many diseases are accompanied by quantitative changes in the composition of serum proteins (disproteinemia). The nature of these changes is taken into account when making a diagnosis and assessment of the severity and stage of the disease.
With the help of the data given in Table. 1.5, make an assumption of the disease for which the electrophoretic profile presented in Fig. 1.36.
Table 1.5. Changing the concentration of serum proteins in pathology
AK are monomeric structural units The protein molecule of which is composed of a polypeptide chain. AK can be in two steric forms: L- and D-. These forms are mirrored symmetrical. In them, the massive lateral radical R and H-atom, which are changing with α-carbon in places. These forms are not only glycine, the side chain of which consists of a H-atom. Side chains are composed of residues L-amino acids, only they are encoded by genes. D-residues are not encoded in matrix protein synthesis, but synthesized by special enzymes. Receit (The transition L-in D-) with biosynthesis, and also spontaneously in proteins is practically not occurring, but often occurs during the chemical synthesis of peptides.
The protein molecule is characterized by the presence of durable covalent and relatively weak non-revalent connections. Such a combination of covalent and non-covalent bonds provides a protein molecule of certain strength and dynamism in the process of functioning (Fig. 1).
a - electrostatic interaction; b - hydrogen bonds; B - the interaction of non-polar side chains caused by pushing the hydrophobic radicals into the "dry" zone of solvent molecules; G is disulfide ties (double curved line denotes the polypeptide bond ridge).
Figure 1 - types of connections in the protein molecule (according to Filippovich).
Covalentcommunication in the protein molecule can be two types - peptide and disulfide.AK in the protein chain is interconnected peptideconnections FROM and N.atoms. Peptide, or acidifaminal connection ( -S-NH-), is an typical covalent bond. Peptide communication occurs when the carboxyl group of one AK and the amino group is different. Free amino and carboxyl groups of the formed dipeptide are able to reinforce polycondensation with new AC molecules, with the formation of a high molecular compound. Thus, with the help of peptide bond, amino acid residues are connected to each other, forming a regular cable molecule, from which a variety of lateral groups depart (R 1 ... R m). The number of units of the side chain (m) is encoded by the genome and ranges from several tens to many thousands. In the process of protein biosynthesis, the residues of individual amino acids with each other in linear sequence occurs:
NH-CH-CO-NH-CH-CO- ... -NH-CH-CO-
Compounds that are formed as a result of condensation of several AK, got a name peptides (di-, tri-, tetrapeptides, etc.). The composition of peptides may include not only proteinogenic, but also non-replaceable AK. Peptides play an important role of intermediate products in metabolism, and many of them are physiologically very active compounds. Peptides are some antibiotics (gramicidine, lichhenoformin), hormones (insulin, oxytacine, vasopressin), toxins (amanitins). Peptides may be a closed polypeptide chain, i.e., be cycloptides, and some even have a bicyclic structure. Among the cyclopepids there are strong toxic substances (poisonous mushroom pale custodia ( Amanita Phalloides.).
The names of the peptides are determined by the names of the AK included in its composition, listed sequentially, starting from the N-terminus, and the suffix -in- in the names of all AK, with the exception of the C-terminal, having a free coxy group (carboxyl), is replaced with suffix -il. For example, if two molecules of alanine and one molecule of glycine are involved in the formation of three peptides, the triceptide is called alaglylanylglycine or alalagali. Abbreviated amino acids are denoted by three-letter symbols (Table 1).
Table 1 - Abbreviated amino acid designations
An important role in stabilizing the spatial structure of the protein molecule is played covalent disulfide bonds (-S-S-), which are formed as a result of oxidation of sulfhydryl groups of cysteine \u200b\u200bresidues. Disulfide bonds can be formed between the residues of cysteine \u200b\u200bof two polypeptide chains or two substances of cysteine \u200b\u200bof one polypeptide chain, while stabilizing a certain conformation of the protein molecule. In stabilization of the conformation of a protein molecule, a significant role is played non-revalent ties and interaction. These include hydrophobic, electrostatic, ion interactions, as well as hydrogen bonds. They maintain the spatial structure of the protein is much weaker than chemical bonds that fix the sequence of monomers (AK) in the protein chain.
Hydrophobic interaction It occurs when the hydrophobic hydrocarbon and aromatic radicals of some amino acids (alanine, valine, leucine, isoleucine, phenylalanine and tryptophan) occurs. The process of hydrophobic interaction can be represented as the movement of non-polar groups of the polypeptide chain (methyl -CH 3, ethyl-С 2 H 5, phenyl -C 6 H 6) from water to hydrophobic areas formed by the association of these groups. Due to this movement, non-polar groups are affected in close proximity to each other in the inner part of the molecule, and the hydrophilic groups are placed on the surface and in contact with water.
Hydrogen bondsthey are formed between hydrogen atoms, covalently connected to an atom containing a watered electronic pair, or another electronegative atom. In biological structures, hydrogen bond is most often formed due to the hydrogen atom associated with oxygen or nitrogen. Hydrogen bonds can be intra and intercepted. Internal hydrogen bonds stabilize α-spirals, and intercellular - β-folded structures.
Ionic (salt) links.They are supposedly formed between dissociated free carboxyl groups (soo) monoaminodicarbonic amino acids (glutamine and aspartic) and protonated free amino groups (NH 3 +) diamino-monocarbonic amino acids. Ionic ties can be intra and intercepted.
Levels of structural organization of the moleculesquirrel. The functional properties of proteins are determined by the sequence of AK and their spatial structure. From this point of view select four levels: primary, secondary, tertiary and quaternary structure.
Under primary structure Understand the qualitative and quantitative composition of AK, as well as their sequence of location in the polypeptide chains of a protein molecule. The protein molecule can have one or more polypeptide chains. For example, the enzyme molecule ribonucleaserepresents one polypeptide chain having eight cysteine \u200b\u200bresidues forming four intramolecular disulfide bonds. The hormone insulin consists of two polypeptide chains associated with disulfide bridges between the residues of cysteine.
The secondary structure shows the spatial configuration of the protein molecule. Three types of secondary structure are distinguished: α-spiral, β-folded and collagen spiral.
In the stabilization of the secondary structure, an important role is played hydrogen bondswhich arise between a hydrogen atom connected to an electro-negative nitrogen atom of one peptide bond, and an amino acid carbonyl oxygen atom, and they are directed along the axis of the spiral. Energy calculations show that the right α-spiral is more efficient (Fig. 2). Fibrigrillary α-keratines (wool, leather, feathers) consist of several polypeptide chains having a right α-helical configuration, and form durable superspirals that perform mechanical functions.
Figure 2 - α-spiral configuration of protein structure
Another type of secondary protein structure, got a name β-folded structureor β-folded layer. In fig. 3 shows a model of such a structure (A - side view, b - top view). Points in the figure shows intercellular hydrogen
Figure 3 - β-folded configuration of protein structure
links. With such a spatial location, the system is formed parallel and anti-parallelly placed fragments of one or several polypeptide chains. Polypeptide chains in layouts are completely elongated. The folds appear due to the fact that the plane of two adjacent peptide bonds form some angle. The system stabilizes due to the transverse hydrogen bonds between the chains, located perpendicular to the orientation of the polypeptide bonds. The distance between the chains is 0.95 nm, and the period of identity along the chain is 0.70 nm for parallel chains and 0.65 nm for anti-parallel. This structure is characteristic of fibrillar proteins (β-keratin, fibroin, etc.). In particular, β-keratin is characterized by a parallel arrangement of polypeptide chains, which are additionally stabilized by intercellular S-S-S-SI-SI-S-bonds. In the fibrine of silk, adjacent polypeptide chains of anti-parallel.
Third Type of Secondary Structure - collagen Spiral. It consists of three spiralized chains having a rod shape with a diameter of 1.5 nm and about 300 nm. Spiralized chains are twisted one around the other and form a superspio. The distance between the two AC residues along the axis of the spiral is 0.29 nm, and one round of the spiral accounts for 3.3 residues. The collagen spiral is stabilized by hydrogen bonds arising between the hydrogen of peptide NH groups of the residues of AK of one chain and the oxygen of co-groups of AK residuals of another chain. Such a structure gives protein high elasticity and durability.
Tertiary structure.Most proteins in native condition have a very compact structure, which is determined by the size, form, polarity of AK radicals, as well as the sequence of AK (Fig. 4). The formation of a native globular structure is a multicomponent process based on different types of non-merant interactions. The conversion of the expanded polypeptide chain into a compact molecule is accompanied by hydrophobic interactions of hydrocarbon radicals such as leucine, isoleucine, phenylalanine, tryptophan, rather removed from each other in the polypeptide chain. Almost all non-polar or hydrophobic radicals These AK are located within the globule and ensure the stability of its structure. Polar or ionic radicals (especially aspartic and glutamic acids, arginine and lysine) are located on the outer surface of the molecule and are in the hydrated state. In places of folds of the polypeptide chain, the remnants of such ak such as proline, isoleucine and series, which are not capable of forming α-spiral structures are localized. Thus, there is a close relationship between the sequence of AK in protein and its conformation. Differences in the amino acid composition and in the sequence of individual AC residues determine the occurrence of local unstable points in the polypeptide chain, in which the stability of the α-helix is \u200b\u200bbroken and bends can be created under the action of various molecular forces.
Figure 4 - Tertiary protein structure
A significant influence on the process of forming a native conformation of a protein or its tertiary structure is hydrophobic and ionic interactions, hydrogen bonds, etc. Under the action of these forces, a thermodynamically appropriate conformation of the protein molecule and its stabilization is achieved. After the process of coagulation of the polypeptide chain is completed, covalent disulfide bonds play an important role in stabilizing its conformation.
Currently, the tertiary structure of myoglobin, hemoglobin, RNA-Ase, lysozyme, chymotrypsin, carboxypeptidases and other proteins has been deciphered.
Under quaternary structure The characteristic method of combining and location in the space of individual polypeptide chains constituting one functionally individual molecule is implied. In the composition and complexity of the primary, secondary and tertiary structure of the subunit can be very different. For example, the hemoglobin molecule consists of four subunits, which are combined into a multimer with a molecular weight of 60000-70000, RNA polymerase from E. coli. It has five subunits, and a tobacco mosaic virus protein contains several thousand identical subunits with a molecular weight of about 17500 each. In the formation of a quaternary structure, hydrogen bonds, electrostatic, van der Wales and hydrophobic interactions participate.
For a quaternary structure of some proteins, the globular location of subunits (hemoglobin) is characterized, other proteins are combined into spiral quaternary structures by type of screw symmetry (tobacco mosaic virus). The quaternary structure is installed for hemoglobin, tobacco mosaic virus, RNA polymerase, lactate dehydrogenase, catalase, aspartate-carboylase, etc.
All processes in the cell are carried out with the participation of proteins. Their functions are extremely diverse. Each given protein as a substance with a certain chemical structure performs one highly specialized function and only in several separate cases - several interrelated.
Going away from the cell to the molecular level we we meet with the following basic features of proteins:
1.Catalogic (enzymatic) function:Numerous biochemical reactions in living organisms flow under mild conditions at temperatures close to 40 ° C, and pH values \u200b\u200bare close to neutral. Under these conditions, the speed of course of most reactions are negligible, therefore special biological catalysts are needed for their acceptable implementation - enzymes.Even such a simple reaction as the dehydration of coalic acid:
CO 2 + H 2 O HCO 3 - + H +
catalyzed by the enzyme carboangeyndrase. In general, all reactions, with the exception of the 2H 2 o®4h + 4e photolysis reaction - + o®4h + + 4e - + o 2, in living organisms catalyzed by enzymes. As a rule, enzymes are either proteins or protein complexes with any cofacitor - metal ion or a special organic molecule. Enzymes have high, sometimes unique, selective action. For example, enzymes catalyzing the addition of A-amino acids to the corresponding T-RNA in the protein biosynthesis process catalyzed the connection of only L-amino acids and do not catalyze the attachment of D-amino acids.
2. Transport function of proteins.Proteins are used for the stocking and transfer of oxygen (hemoglobin, hemocyanin). This feature resembles enzymatic, but it is different from it, because O 2 does not undergo changes.
Inside the cells should flow numerous substances that ensure it with building materials and energy. At the same time, all biological membranes are constructed according to a single principle - a double layer of lipids in which various proteins are immersed, and the hydrophilic portions of macromolecules are concentrated on the surface of the membrane, and the hydrophobic "tails" is in the thickness of the membrane. Such a structure is impenetrable for such important components, like sugar, amino acids, alkali metal ions. Their penetration of the cell is carried out with the help of special transport proteins mounted in the cell membrane.
3. Regulatory functions - low molecular weight polypeptides (insulin, oxytocin), hormones stimulate functional activity in cells of other tissues and organs.
4. Protective immunological function. The immune system has the ability to respond to the appearance of alien particles to develop a huge number of lymphocytes that can specifically damage these particles, which can be alien cells, such as pathogenic bacteria, cancer cells, oxolecular particles, such as viruses, macromolecules, including alien proteins. One of the groups of lymphocytes - In lymphocytes, produces special proteins allocated to the circulatory system, which recognize alien particles, forming a highly specific complex at this stage of destruction. These proteins immunoglobulins Higher organisms protect them from alien biopolymers due to their specific structure (functional group).
5. Storage function, chemical and electrical transmission.
6. Structural function. Along with proteins that perform thin highly specialized functions, there are proteins that have mainly structural meaning. They provide mechanical strength and other mechanical properties of individual tissues of living organisms. First of all it collagen - The main protein component of the extracellular matrix of the connective tissue. Mammals collagen is up to 25% of the total mass of proteins. In elastic tissues - skin, walls of blood vessels, lungs - In addition to collagen, extracellular matrix contains protein elastincapable of stretching quite widely and return to its original state.
Another example of structural protein - fibroin Silk, secreted by silkwood caterpillars during the formation of a pupa and being the main component of silk yarns.
7. Motor proteins. Muscular reduction is a process, during which the transformation of chemical energy, stored in the form of macroeergic pyrophosphate bonds in ATP molecules, into mechanical work. Direct participants in the reduction process are two proteins - Aktin and Miosin.
8. Receptor function. Of great importance, especially for the functioning of multicellular organisms, have proteins receptorsmounted in plasma membrane cells and employees for perception and transformation of various signals entering the cell from both the environment and from other cells. As the most studied can be brought acetylcholine receptorsThe cell membrane in a number of cross-line contacts, including in the cerebral cortex, and neuromuscular compounds. These proteins specifically interact with acetylcholine and responds to this transmission of the signal inside the cell. After receiving and converting the signal, the neurotransmitter must be removed so that the cell is prepared for the perception of the next signal.
9. Toxins: A number of living organisms as protection against potential enemies produce highly poisonous substances - toxins. Many of them are proteins, however, there are among them and complex low molecular weight organic molecules. As an example of such a substance, the poisonous beginning of the pale urban - a-Amanitin:This compound specifically blocks the synthesis of eukaryotic and-RNA. For a person, a deadly dose is a few mg of this toxin.
Primary and secondary protein structure. Proteins are not static formations. These are structures that can undergo certain conformational changes in the biological functioning process. Analysis of the conforms is carried out on the basis of various levels of the organization of protein molecules. Back in 1959, K.Liderstrem-Lang allocated four levels of the structural organization of proteins - primary, secondary, tertiary and quaternary structure. Later, on the basis of comparison of data of x-ray structural analysis, calorimetry and other methods, two more organizations were allocated - super-control structures and protein domains.
Amino acid sequence is called primary protein structure. The study of the arino acid location in proteins is an important stage in the study of the protein structure. Currently, this analysis is carried out automatically using devices of sepolenitors. In recent years, a new method for determining the amino acid sequence is used. The DNA fragment containing the structural gene of this protein is isolated, decipher the sequence of nucleotides and translate it according to the genetic code into the amino acid sequence. The primary structure is a one-dimensional representation of a protein molecule. Knowledge of the primary structure is used to predict the secondary and tertiary protein structure. The simultaneous use of amino acid sequence and crystallographic maps of electron density allows you to restore the spatial location of all atomic groups in protein.
In the polypeptide chain, the peptide group is flat and rigid. The polypeptide chain can be represented as a sequence of the same type of planes (peptide groups) interconnected with single connections. Rotation around these ties is not completely free due to steric restrictions. The angle of rotation around the bonds of C - C A is denoted by ψ, and the angle of rotation around the connections n - with a denote φ. G.Ramachandran conducted calculations of the conformational states of the polypeptide chain using a computer and identified the area of \u200b\u200bpossible values \u200b\u200bof ψ and (Ramacandrane graphics or conformational cards). On conformational maps, the values \u200b\u200bof the angles ψ and φ in proteins are not arbitrary, they are clearly limited to specific areas, which indicates the existence of a limited number of conforms of the polypeptide chain.
Under the secondary structure of the protein understand the ordered location of the polypeptide chain stabilized by hydrogen bonds between peptide groups. Considering this structural level, they indicate the local conformation of the sections of the polypeptide chain. Often meets and the most energy and sterically favorable secondary structure is the right α– spiral, which was first postulated by L. Polying and R. Kori (1951). The most important characteristics α– spirals: 1) The number of amino acid residues per step of the spiral is 3.6; 2) Spiral pitch d \u003d 0.54 nm; 3) broadcast for one residue along the spiral Δd \u003d 0.15 nm; 4) Radius α– spirals r. \u003d 0.23 nm; 5) Hydrogen bonds (parallel axes of the spiral) are formed between each first and fourth peptide group; 6) for α– spiral φ \u003d -57 ° and ψ \u003d -47 °. As can be seen from cross cut α– spirals on each turn of it takes place to be right to 60 °. As a result of such a shift, only 10 revolutions 1st peptide group will definitely coincide with the 36th peptide group.
Secondary structures of protein molecules are parallel and anti-parallel β-folded sheets (or β-structure). On the conformation map of Ramacandrane for the β-layer with anti-parallel chains φ \u003d -139 ° and ψ \u003d + 135 °, for the β-layer with parallel chains φ \u003d - 119 ° and ψ \u003d + 113 °. Most of they have no more than six polypeptide chains stabilized by hydrogen bonds, and six amino acid residues along the length of each chain. Dimensions of such a sheet: Width T \u003d 2.5 nm, Length L \u003d 2.0 nm. Most folded sheets have a twisted form. Twisting goes perpendicularly elongated chains.
The next level of the organization of protein molecules are super-functional structures. An example of such structures is superspiral structures. In them two α– spirals (in tropomyosine, light meromozine, paramyosine) or three α - Spiral (in fibrinogen) twisted relative to each other. Superpiral step in a light meromozine is α= 18.6 nm. On the example of tropomyosis with a well-known amino acid sequence, it was concluded that the superspio is stabilized by hydrophobic interactions between individual α - spirals.
Primary chain structure and the formation of protein globule
One of the most important problems of protein physics is the problem of communication between the primary structure of the polypeptide chain and the spatial structure of the globule. The biologically functional native spatial structure of macromolecules, and the primary structure is genetically encoded. And why the protein molecule forms a globe, otherwise, why is the protein capable of self-assembly and protein in this state can already perform its functions? As the GUTSCO was installed, the specific location of amino acids matters for the spatial structure of the protein. The amino acids "Unciral" distinguish can not form spirals and "spirals" - can bend (ASP, CIS, TIR, Ser). Depends on the twist, laying the molecule. And especially important for the formation of the spatial structure of the protein has an amino acid glycine - this is how a universal hinge can occupy a variety of positions.
Currently it is assumed that the self-organization of the protein globule, there is no result of a certain directional process. Many researchers believe that the error-free self-erroxization program is encoded in the most primary structure. Self-organizing stadia occurs, so that every next stage is formed an increasingly complex and stable structure.
Regular conformations of polypeptide chains stabilized with hydrogen bonds ( α and β - forms) are resistant only under certain conditions, a change in temperature, pH, the solvent of the medium leads to the transformation transitions. Ameriknets Doti found that the transitions of the spiral-tangle proceed in a very short time. The transition is characterized by a change in viscosity, light scattering, etc. The sharpness of the transition indicates a cooperative character, i.e. Each unit of the macromolecule is in fixed state with hydrogen bonds. Under the action of foreign factors, the packaging of molecules occurs, i.e. Conformation.
According to a scientist, the first stage in the expanded protein chain is formed, fluctuating (modified, non-permanent) embryos of spiral sections with an elongated structure (image location) are formed. In the second stage, one or more germ pairs are combined, forming the centers of the organization of the tertiary structure. In the third stage, the centers are growing due to the attachment of neighboring regions of the chain.
And at the last, the fourth stage is formed a single compact structure of the globule by growth or combining several centers.
Domains and Tertiary Squirrel Structure
The tertiary protein structure is the thermodynamically the most stable form of coagulation and laying the polypeptide chain. The question arises, how does the protein coagulation occur, how does one-dimensional information laid in the amino acid sequences are implemented in spatial information? Experiments on denaturation and renaturation of proteins have shown that the processes of destruction and formation of a compact tertiary structure pass quite quickly: the nuclease staphylococci is re-coated for 1 s.
To explain the coagulation process, a nucleation model is used. In this model, it is assumed that the short segments of the polypeptide chain are very quickly coagulated independently of each other, and at the second stage they come closer, forming a compact three-dimensional structure. Protein segments form α - Spired and β-layers at high speed. Experimentally shown that transitions Spiral - Tangle pass during 10 -6 to 10 -8 c.
Recently, another important level of a structural organization has been highlighted in proteins. An analysis of the electronic density of proteins with molecular weight more than 20,000 showed that proteins consist of several globular areas, weakly connected. These areas received the name of the domains. Individual domains can often be isolated from protein using proteolytic enzymes without losing them functional properties. The domain implies the area of \u200b\u200bone polypeptide chain concluded in the compact volume. These are areas of chains that are coagulated and they are deployed in protein independently of each other.
Domain can be viewed as a relatively autonomous structural unit. With the help of scanning microcalorimetry, privov showed the presence of individual cooperative blocks in complex proteins, for which jump-shaped structural transitions are characterized with thermal denaturation. It turned out that in many cases, such cooperative proteins well correspond to the dedicated proteolytic fragments of proteins. This made it possible to identify cooperative blocks with protein domains. Frequently isolated proteolytic fragments has structural properties similar to cooperative blocks, i.e. Their melting points and enthalpy of transitions are coincided, as well as they retain the functional characteristics of native proteins. Domains are interconnected by a very limited amount of peptide bonds, which are relatively easily broken under the action of proteolytic enzymes.
Currently, with the help of scanning microcalkorimetry of electron microscopy, proteolytic splitting establishes a domain structure in such high molecular weight proteins as immunoglobulin, myozic, fibrinogen, etc.
Domains may be an important intermediate formation in the process of coagulation of the native protein structure. Squirrels consisting of domains must have a more flexible structure than proteins in which various sections are fastened with each other. Apparently , reversible conformational changes affecting the function of enzymes are associated with interety perestroins without changing the structural stability of the domains themselves.
The hypothesis of the molten globule.One method of studying the folding of the polypeptide chain into a three-dimensional structure is denaturation and subsequent renaturation of the protein molecule.
Experiments from K. Anfinsen with ribonuclease definitely show the possibility of assembling exactly the spatial structure that was violated as a result of denaturation.
In this case, the restoration of native conformation does not require any additional structures. What are the coagulation models of the polypeptide chain about the appropriate conformation are the most likely? One of the common protein self-organization hypotheses is the hypothesis of the molten globule. As part of this concept, several stages of protein self-assembly are distinguished.
1. In the deployed polypeptide chain using hydrogen bonds and hydrophobic interactions, separate areas of the secondary structure are formed, which seems to be seeded to form full secondary and super-functional structures.
2. When the number of these areas reaches a certain threshold value, the lateral radicals are reorienting and the transition of the polypeptide chain into a new more compact shape, and the number of non-covalent bonds increases significantly. A characteristic feature of this stage is the formation of specific contacts between atoms located on remote areas of the polypeptide chain, but those convertible as a result of the formation of the tertiary structure.
3. At the last stage, the native conformation of the protein molecule is formed, associated with the closure of disulfide bonds and the final stabilization of the protein conformation. Non-specific aggregation of partially rolled polypeptide chains is also not excluded, which can be qualified as native white formation errors. Partially rolled polypeptide chain (stage 2) called molten globule, and stage 3 it is the slowest in the formation of a mature protein.
In cells there are a number of catalytically inactive proteins, which, nevertheless, contribute a great contribution to the formation of spatial protein structures. These are the so-called chapironins and shaps. One of L. Ellis Molecular Chapironov's Molders Calls them with a functional class not related to each other of proteins families that help the correct non-rigorous assembly of other polypeptide-containing structures in vivo, but are not part of the collected structures and do not participate in the implementation of their normal physiological functions.
Chapirona helps the correct assembly of three-dimensional protein conformation by forming reversible non-rigal complexes with a partially rolled polypeptide chain, at the same time inhibiting incorrectly formed bonds leading to the formation of functionally inactive protein structures. The list of functions inherent in champions includes sewn of molten globes from aggregation, as well as the transfer of newly incident proteins to various cell loci. Chapirona is preferably proteins of heat shock, the synthesis of which is sharply enhanced with stressful temperature effects. The families of these proteins are found in microbial, plant and animal cells. The classification of chapironov is based on their molecular weight, which varies from 10 to 90 kda. Basically, the functions of chapironov and chaspironins differ, although those and others are proteins-assistants of the processes of education of the three-dimensional structure of proteins. Chapirona hold a new-seated polypeptide chain in the deployed state, not allowing it to turn into different from the native form, and shaponins provide conditions for the formation of the only correct, native protein structure.
Quaternary structure of proteins
The formation of chaotic formed aggregates is an error that leads to the appearance of functionally inactive proteins, therefore, the cells are provided for the mechanisms of rapid degradation and decay to separate amino acids. However, in nature there are many genetically deterministic aggregates, including several polypeptide chains forming large protein macromolecules. Quaternary structure is called associated two or more subunits oriented in space. Apparently, more correctly in relation to the Quaternary structure of proteins, they are not talking about aggregates, but about the ensembles global. Describing the quaternary structure of proteins, its pseudovariants should be excluded. So the protein hormone insulin consists of two polypeptide chains, but they are not full globes, and are formed as a result of limited proteolysis of a single polypeptide chain. Are not proteins with a true quaternary structure and multimenme complexes. They are typical supramolecular structures. In the formation of a quaternary structure, individual subunits interact with each other solely with the help of non-melative bonds, primarily hydrogen and hydrophobic. It is very significant that the contact surfaces of interacting subunits are complementary to each other. In the contact sites there are hydrophobic groups that were called "sticky stains".
The mutual orientation of electronegative atoms, facilitated by the presence of complementary sites, contributes to the formation of a large number of hydrogen bonds. This ensures the realization of the cooperative effect and the stabilization of the macromolecule. In addition, the multiplicity of non-covalent bonds is the basis of the transfer of structural rearrangements from one subunit to others.
Proteins having a quaternary structure are often called oligomeric. Distinguish homomericand heteromericproteins. Gomemaker includes proteins that have all subunits have the same structure. As an example, you can catalase protein consisting of four completely equitable subunits. In heteromeric proteins, individual subunits not only differ in the structure, but also can perform various functions. For example, a RNA polymerase protein consists of five subunits of various buildings and with unequal functions.
Proteins are polypeptides, the molecular weight of which exceeds 6000-10000 Daltons. They consist of a large number of amino acid residues.
Unlike low molecular weight peptides, proteins have a well-developed three-dimensional spatial structure, which stabilizes various kinds of strong and weak interactions. There are four levels of the structural organization of a protein molecule: primary, secondary, tertiary and quaternary structures.
Primary protein structure is a sequence of amino acid residues interconnected by peptide bonds.
For the first time, the role of peptide bonds in the construction of protein molecules was put forward by the Russian biochemist A. Ya. Danilevsky, the ideas of which were formed the basis of the polypeptide theory of the structure of proteins, formulated by the German chemist E. Fisher in 1902
The basis of the primary structure of the protein molecule forms a regularly repeated peptide cable - NH-CH-CO-, and the lateral radicals of amino acids make up its variable part.
The primary protein structure is durable, because its constructions are based on peptide bonds, which are strong interactions;
Connecting each other in various sequences, proteinogenic amino acids form the isomers. Of the three amino acids, you can build six different tripipeptides. For example, glycine, alanine and valine - Gly-Ala Val, Gly Val-Ala, Ala-Gly Val, Ala-Val-Gly, Val-Gly-Ala and Val-Ala-Gly. Of the four amino acids, you can form 24 tetrapeptide, and from five to 120 pentapeptides. Of 20 amino acids, you can construct 2 432 902 008,176,640,000 polypeptides. At the same time, each amino acid is used in the construction of the considered polypeptide chains only once.
Many natural polypeptides are in their composition hundreds and even thousands of amino acid residues, and each of the 20 proteinogenic amino acids can occur in their composition repeatedly. Therefore, the number of possible variants of polypeptide chains is infinitely large. However, in nature, not all theoretically possible variants of amino acid sequences are being implemented.
The first protein, the primary structure of which was decrypted, is the bullish insulin. Its molecule consists of two polypeptide chains, one of which contains 21, and the other - 30 amino acid residues. Chains are connected to each other disulfide bonds. Another disulfide bond is located inside a short chain. The sequence of the amino acid residues in the insulin molecule was established by the English biochemist F. Sanger in 1953
Thus, F. Sanger confirmed the polypeptide theory of the structure of the protein molecule E. Fisher and proved that proteins are chemical compounds with a certain structure that can be depicted using a chemical formula. To date, the primary structures of several thousand proteins are deciphered.
The chemical nature of each protein is unique and closely related to its biological function. The ability of the protein to perform the function inherent in it is determined by its primary structure. Even small changes in the sequence of amino acids in protein can lead to a serious impairment in its functioning, the occurrence of severe disease.
Diseases associated with violations of the primary structure of the protein were called molecular. To date, several thousand such diseases are opened.
One of the molecular diseases is a sickle cell anemia, the cause of which lies in the violation of the primary hemoglobin structure. In people with a congenital anomaly, the structure of hemoglobin in a polypeptide chain consisting of 146 amino acid residues, in the sixth position there is valine, whereas in healthy people in this place - glutamy acid. Anomalous hemoglobin is worse than transporting oxygen, and the blood erythrocytes of patients have a sickle form. The disease is manifested in slowing development, the general weakness of the organism.
Primary protein structure is given genetically. This makes it possible to organisms of one species to maintain constancy of a set of proteins. However, in different types of living organisms, proteins that perform the same function are not identical to the primary structure - in some sections of the polypeptide chain, they may have unequal sequences of amino acids. Such proteins are called homologous (Greek. "Homology" - consent).
Studies of con formations of protein molecules have shown that the polypeptide chains are not pulled out strictly linearly, and in a certain way collapsed in space, forming a secondary structure.
The secondary protein structure is a combination of ordered and amorphous sections of the polypeptide chain.
Studying the crystal structures of compounds containing amide groups, the American biochemist L. Pauling found that the length of the peptide bond is close to the dual bond length and is 0.1325 nm. Therefore, the free rotation of carbon and nitrogen atoms around the peptide bond is difficult.
In addition, the atoms of peptide groups and α-carbon atoms are located in the polypeptide chain in approximately one plane. In this regard, turns in the polypeptide chain can only be performed on bonds adjacent to carbon atoms.
Due to the turns of peptide groups around α-carbon atoms, as L. Poling and R. Corey installed in the early 50s of the last century, the polypeptide chain is folded into the α-spiral and stabilizes due to the formation of the maximum possible number of hydrogen bonds.
In the formation of the secondary structure of the protein molecule, hydrogen bonds arise between the atoms of peptide groups located on the adjacent turns of the OS-spiral against each other. A hydrogen atom connected by covalent bond with nitrogen atom has some positive charge. An oxygen atom connected by double bond with carbon atom has some negative charge. The hydrogen atom, being opposite the oxygen atom, is associated with a hydrogen bond. Hydrogen bond is weak. However, due to the formation of a large number of these links, a strictly ordered structure is preserved.
Hydrogen bonds are always directed parallel to the imaginary A-helix axis, and amino acid radicals - outward from its turns. Peptide groups are combined with hydrogen bonds mainly through four amino acid residues, since it is their O-C- and H-N-groups that turn out to be spatially concentrated.
A-spiral is human rights. If you look at it from the end, from the N-end side, the tightening of the polypeptide chain is clockwise. Installed A-Spiral Parameters. The distance between adjacent turns (spiral pitch) is ∅54 nm, and the inner diameter of the helix is \u200b\u200b1.01 nm. One full spiral round includes 3.6 amino acid residues. The complete repetition of the structure of the α-helix occurs every 5 turns, including 18 amino acid residues. This segment of α-helix is \u200b\u200bcalled a period of identity and is 2.7 nm in length.
The polypeptide chains are folded into the A-Spiral not all over its entirety. The percentage of surplus plots in the protein molecule is called degree of spiralization. Proteins differ significantly according to the degree of spiralization, for example: for hemoglobin of blood, it is very high - 75%, for insulin is also quite high - 60%, for albumin chicken eggs, is much lower - 45%, and for chymotrygenogen (inactive precursor of the digestive enzyme) is extremely low - Just 11%.
Differences in the degree of spiralization of proteins are associated with a number of factors that prevent the regular formation of hydrogen bonds between peptide groups. In particular, the formation of disulfide bonds cysteine \u200b\u200bresidues connecting various sections of one or more polypeptide chains. In the region close to the residue of the imino acid of the Proline, around the α-carbon atom of which it is impossible to rotate the adjacent atoms, the bend is formed in the polypeptide chain.
A series of proteinogenic amino acids possess such radicals that do not allow them to take part in the formation of the α-helix. These amino acids form parallel folds, connected with each other hydrogen bonds. This type of regular section of the polypeptide chain was called the structure of a folded layer, or β-structure.
In contrast to the A-helix, having a rod shape, the β-structure has a shape of a folded sheet. It is stabilized by hydrogen bonds arising between peptide groups located on the adjacent segments of the polypeptide chain. These segments can be directed either in one direction - then a parallel β-structure is formed, or in opposite - in this case an anti-parallel β-structure occurs.
Peptide groups in the β-structure are located in the folds of the folds, and the lateral amino acid radicals - above and under the planes. The distance between adjacent areas of the polypeptide chain in the structure of the folded layer is 0.272 nm, which corresponds to the length of the hydrogen bond between the groups - and -nh-. The hydrogen bonds themselves are perpendicular to the direction of the folded layer structure. The content of β-structure in various proteins varies widely.
Some sections of polypeptide chains do not have any ordered structure and are randomballs. Such sites are called amorphous (Greek. "Amorphos" is informal). However, in each protein, amorphous areas have their fixed conformation. At the same time, in contrast to relatively rigid areas - α-helix and β-structures - amorphous tangles can relatively easily change their conformation.
Proteins differ in the content of different types of secondary structure. For example, only α-helix were found in the hemoglobin structure. In many enzymes there are various combinations of both α-helix and β-structures, among immunoglobulins there are proteins that have only a β-structure. Finally, there are also such proteins in which ordered areas are present in minor quantities, and most of the polypeptide chain has an amorphous structure.
The polypeptide chains with an formed secondary structure are defined in space, creating another level of the structural organization of the protein molecule - the tertiary structure.
The tertiary protein structure is formed as a result of a specific laying of ordered and amorphous sections of the polypeptide chain in some volume of space. It is maintained due to strong and weak interactions arising between the side radicals of amino acid residues. Silent interactions include disulfide bond, and weak is hydrogen and ionic communications, as well as hydrophobic interactions.
Disulfide bond is formed by the interaction of two closely arranged cysteine \u200b\u200bresidual radicals containing free sulfhydryl groups.
Disulfide bridges can be interconnected by not only individual sections inside one polypeptide chain, but also (in the formation of a quaternary protein structure) various polypeptide chains.
Hydrogen bond can occur between the side radicals of the residual amino acids containing on-groups, for example, between two serine residues.
In addition to the radicals of serine residues, in this way, hydrogen bonds can form trearone and tyrosine residue radicals.
In the formation of the tertiary structure of the protein molecule, many hydrogen bonds arising between side radicals, for example: tyrosine and glutamic acid, asparagine and serine, lysine and glutamine, etc.
Ionic bonds occur when the negatively charged radicals of the residues of acid amino acids - asparty or glutamic - with positively charged radicals of the residues of the main amino acids - lysine, arginine or histidine. The ion connection between the radicals of the residues of aspartic acid and lysine.
Hydrophobic interactions occur in water, due to attraction to each other, non-polar radicals of amino acid residues. For amino acids with non-polar radicals, for example, alanine, valine, leucine, isoleucine, phenylalanine, methionine are belonging to. Hydrophobic interaction between the side radicals of valine and alanine residues.
To avoid contact with water, non-polar radicals of amino acid residues seek to get together inside the protein molecule. The protein turns into a compact body - globe (lat. "Globulus" - ball). Inside the globule, a hydrophobic kernel is formed, and the polar radicals of amino acid residues are located outside, which interact with water. Polar radicals have, for example, acidic and basic amino acids, series, threonine, tyrosine, asparagin, glutamine.
Thus, each protein globule is surrounded by a hydrate shell represented by the so-called "water fur coat", which also includes structured water molecules that can hold on the surface of the globule to half the hydrophobic radicals available in the polypeptide chain. This causes the solubility of the protein.
Due to the set of interradical interactions, the individual areas of the protein molecule are spatially converted and recorded relative to each other. In the course of the formation of the tertiary structure of the protein, its active center is formed. As a result, the protein acquires the ability to perform its biological function.
The first protein, the tertiary structure of which was installed, is myoglobin.
Tertiary globuses can interact among themselves so that a single molecule arises. Such globulas are called subunits, and their association is a quaternary structure of a protein molecule.
The quaternary protein structure can be built from a different number of subunits held together, mainly due to weak interactions. She is inherent in many proteins.
Subunits, characteristic of the space located in space relative to each other, form an oligomeric (multimeric) complex. The ability of proteins to the formation of such structures allows us to combine several active centers into a single integer centers and interrelated functions, which is very important to ensure the flow of complex metabolic processes.
Quaternary protein structures can be built from 2, 4, 6, 8.10, 12, 24 or more subunits and rarely from the odd number. For example, the quaternary structure of hemoglobin form four pairwise identical subunits.
The quaternary structure of the protein molecule is the same unique as other structures. At the same time, the entire three-dimensional packaging of the polypeptide chain in space is determined by its primary structure. Specific spatial structure (conformation) in which protein molecules have biological activity, called navia (Lat. Nativus - congenital).
Proteins - These are high molecular weight biopolymer organic compounds whose monomers are amino acids. Proteins were highlighted in a separate class of biological molecules in the XVIII century. As a result of the works of the French Chemist A. De Furkrua. First described proteins and offered the name proteins that in modern understanding means protein, Dutch chemist E. Ya. Burtsellius. The first selection of protein (in the form of gluten) from wheat flour was carried out by Ya. Beccari. A feature of the research of proteins beginning of the XXI century. Simultaneous production of data on protein composition of whole cells, tissues or organisms, which deals with separate science - proteomika .
Molecular weight of proteins From 5000 to 150000 and more.
One of the largest single proteins is titin (Component of muscle sarcomers), containing more than 29 thousand amino acids and has a molecular weight of 3000000 yes. But the largest proteins (more than 40000,000 yes) are characteristic of viruses.
Chemical composition . Consist of proteins C. C, η, O, N ; In some proteins is S. , part of the proteins forms complexes with other molecules that contain R, FE, Zn, Cu . Proteins are biopolymers from 20 different monomers - natural major amino acids. Proteins can form interpolymer complexes with carbohydrates, lipids, nucleic acids, phosphoric acid, etc.
Physiochemical properties. Due to the presence of free amino groups and carboxyl groups, proteins are characterized by all properties of acids and bases ( amphoteric properties). The dissociation of amino acids and carboxyl protein groups determine the electrophoretic protein mobility. At low pH values \u200b\u200bof the protein solution, positively charged amino groups are dominated, so proteins are in cationic form. At high pH values, negatively charged coxy groups and proteins will be in an anion form. With some intermediate pH of the amino group and carboxyl groups can interact with each other, then the amount of charges are zero, and the proteins remain fixed in the electric field ( electrical properties). High molecular weight has protein solutions of properties characteristic of colloidal systems, namely: the ability to form gels, high viscosity, low diffusion rate, high degree of swelling, due to which they bind about 80-90% of all water in the body ( colloidal properties). The decay of proteins occurs under the action of acids, alkalis or specific hydrolyz enzymes, which split them to peptides and amino acids. Synthesis is carried out with amino acids with a matrix principle with the help of information RNA. Under the influence of various cleaning, proteins can be praised and falling into a precipitate, losing natural properties. The absence of charge and hydrate shell contributes to the rapprochement of protein molecules, their sticking and falling into the precipitate. This phenomenon is called coagulation It can be reverse and irreversible. The irreversible coagulation can be considered as denaturation of proteins. Denaturation is the process of violation of the natural structure of proteins. This reduces the solubility of the protein, the form and dimensions of molecules and other are changing. The process of denaturation is reversible, that is, the return of normal conditions is accompanied by
{!LANG-74cc9c82943049af3ff57e19b0ab5452!} {!LANG-d60d696af2bf64a91a721d222d148fd2!} . {!LANG-96b7b99ab5c428027f1ba9da29e7be53!} {!LANG-05df280db89aec9648c8844c046c950c!} . {!LANG-0c6a6f08327bbe2d88ff2923dac59f9d!}
{!LANG-80ef09bf5ea20d461d3d896fa46ad946!} {!LANG-6a1c4623451e88c9e07458b3073c7ee3!} {!LANG-87fcdf053b587a9931273524387420c5!}{!LANG-4c6dfd2c49ab7b6fd52072d769697a20!} {!LANG-697623bdd6301319127cd15ef5e08cd1!}{!LANG-61dd83ebeb07e1d50023022e6c9f238c!}
{!LANG-0ca627b6161a5ea2c117699dc331369d!}
{!LANG-2f9a8d8057b8f9ee92eebce7a4be59e2!}{!LANG-4bd7d4fe75f60ebd0376a264a76e3c83!} {!LANG-72db79a0fd0c18f3d75c47417d15cd4e!}{!LANG-19bc663960ceadc0be2b5568252ef01c!} {!LANG-88caab0f4ebf3ff83b849361d5acaa33!}{!LANG-1285ababc77a56b21e31c0061cf839e4!} {!LANG-5dc5567c31040d6b336de083a796abfe!}{!LANG-e6d96e1d24593bae9c2385aa7944e147!} {!LANG-64661d672c8827bc4603773d3c8a66c3!}{!LANG-df4571adc864c996146406d75f9af20f!}

{!LANG-ea6cb923b02a7e28500b315fe238f521!} {!LANG-b02c4e79078c1c7bd63dac40e5b41903!}{!LANG-f37e0a9563156c7cfe306ca5e3b876f6!} {!LANG-c6668595c514f786a5f2c1dc2076b8c9!}{!LANG-be6e9da311dd88bff1d04a6bddc371ec!}
{!LANG-d3ed9e0ce359fd4a772223b625b66388!} {!LANG-441352e853f7436584366890a46ed35a!}{!LANG-c794fe320840f875be456d046bb348d9!} {!LANG-6f66f2644e7982223172c735304bd8a7!}{!LANG-75a6e9ecb20acdfeccf83ac0ead072e6!} {!LANG-d29525abd9e252b0a26a15085c0f7881!}{!LANG-3f329d93e6ebb6e5f959bcc5731f1bfe!}
{!LANG-2195b49ba582b5fc536204d730696dfe!} {!LANG-38e8b7f66f8f7746affa25f0fcbe45e3!}
{!LANG-0e489141f401abb50760ad0fc97c924e!} - {!LANG-cad5adaee1899fca37385f9046be1d04!} 2 {!LANG-657c85026456b6607365c83020d2aed1!}{!LANG-b0d10c6b7d4680aa9bd38abd6e757903!} {!LANG-1cd716a67aa4806e9848e78c85b25ca4!}{!LANG-80da7429645d977fac1a7d729990acf5!}
{!LANG-f1dbf203400d2a0c952db3a76025348e!} ({!LANG-a7fa15053f471c9e9dc3b0af35ed2f7b!}) {!LANG-a05a4eece4dfb61d04c18abf1d7758b2!}{!LANG-1150cd1d884f86989f518c27f8dd665c!} {!LANG-577ffc0b1f2c22c266b29e9e40745215!}{!LANG-40621674a2489479cfacaa76179d83bb!}
{!LANG-284af5ff0eb640e1cbd92bd09ed71244!} - {!LANG-5658f3cd408230d5d2689492511ac578!}{!LANG-84cc719e5c020a5a04f28df138453a43!} {!LANG-668b57cbec566e58c2d682cde8199ccc!}{!LANG-fba776ced9e0a08576799d475f97b36d!}
{!LANG-a1c85dadaf6adc10f16d3adb55d05022!}{!LANG-0fd714f50c63a3fa082331b6c9307c5e!} {!LANG-1f3f853a6a11491791d33c508cd95139!} 3+ -) {!LANG-9d19b6ffdc2a50128dde87d9b102b805!}
{!LANG-40f1c2a8aaf7481b0d56ea0a541879e9!} - {!LANG-448f80fe6d4deb69b4f6ff2a11b250a9!}{!LANG-7d9959ce4bc2abdd55820fe06ab28a10!} {!LANG-fd551d6538ba7eafcce06e9cc555eb10!}{!LANG-b4d38281695d6d967ece9c8062368963!}
{!LANG-fc9a7d10184c05b8f76e76ce786fe458!} {!LANG-dfada37cd14df26cd093296b25c26bc5!} ({!LANG-031cd69915c72c35372c036dad0885f0!}) {!LANG-efe27c4d1d317ed37634bbc6ad4ce372!}) {!LANG-ce688eb267b3c52245bc6c7342898b36!}) {!LANG-16d3898617ef811d8d72ea7f01fd6d1b!} - {!LANG-c9dc78417a1f23fe54f8553df6ee5d35!} 2+ {!LANG-b234042a9258365d5a0cdf8bae899579!} ({!LANG-b51c21380c7cf5477f9aa5c4e988c09c!}) {!LANG-b66c8535dfdc9d9a1584da845ff718c1!}








