Carbon
In the free state, carbon forms 3 allotropic modifications: diamond, graphite and artificially produced carbyne.
In a diamond crystal, each carbon atom is linked by strong covalent bonds to four others placed around it at equal distances.
All carbon atoms are in a state of sp 3 hybridization. The atomic crystal lattice of diamond has a tetrahedral structure.
Diamond is a colorless, transparent, highly refracting substance. It has the greatest hardness among all known substances. Diamond is brittle, refractory, conducts heat poorly and electricity. The small distances between neighboring carbon atoms (0.154 nm) determine the rather high density of diamond (3.5 g/cm3).
In the crystal lattice of graphite, each carbon atom is in a state of sp 2 hybridization and forms three strong covalent bonds with carbon atoms located in the same layer. Three electrons of each carbon atom participate in the formation of these bonds, and the fourth valence electrons form n-bonds and are relatively free (mobile). They determine the electrical and thermal conductivity of graphite.
The length of the covalent bond between neighboring carbon atoms in the same plane is 0.152 nm, and the distance between C atoms in different layers is 2.5 times greater, so the bonds between them are weak.
Graphite is an opaque, soft, greasy to the touch substance of gray-black color with a metallic sheen; conducts heat and electricity well. Graphite has a lower density compared to diamond and easily splits into thin flakes.
The disordered structure of fine-crystalline graphite underlies the structure various forms amorphous carbon, the most important of which are coke, brown and black coals, soot, activated (active) carbon.
This allotropic modification of carbon is obtained by catalytic oxidation (dehydropolycondensation) of acetylene. Carbyne is a chain polymer that has two forms:
С=С-С=С-... and...=С=С=С=
Carbyne has semiconducting properties.
At ordinary temperatures, both modifications of carbon (diamond and graphite) are chemically inert. Fine-crystalline forms of graphite - coke, soot, activated carbon - are more reactive, but, as a rule, after they are preheated to a high temperature.
1. Interaction with oxygen
C + O 2 = CO 2 + 393.5 kJ (in excess O 2)
2C + O 2 = 2CO + 221 kJ (with a lack of O 2)
Coal combustion is one of the most important sources of energy.
2. Interaction with fluorine and sulfur.
C + 2F 2 = CF 4 carbon tetrafluoride
C + 2S = CS 2 carbon disulfide
3. Coke is one of the most important reducing agents used in industry. In metallurgy, it is used to obtain metals from oxides, for example:
ZS + Fe 2 O 3 = 2Fe + ZSO
C + ZnO = Zn + CO
4. When carbon interacts with alkaline and alkaline earth metals the reduced metal combines with carbon to form a carbide. For example: 3S + CaO = CaC 2 + CO calcium carbide
5. Coke is also used to produce silicon:
2C + SiO 2 = Si + 2СО
6. If there is an excess of coke, silicon carbide (carborundum) SiC is formed.
Production of “water gas” (gasification of solid fuel)
By passing water vapor through hot coal, a flammable mixture of CO and H 2, called water gas, is obtained:
C + H 2 O = CO + H 2
7. Reactions with oxidizing acids.
Activated carbon or charcoal, when heated, reduces the anions NO 3 - and SO 4 2- from concentrated acids:
C + 4HNO 3 = CO 2 + 4NO 2 + 2H 2 O
C + 2H 2 SO 4 = CO 2 + 2SO 2 + 2H 2 O
8. Reactions with molten nitrates alkali metals
In KNO 3 and NaNO 3 melts, crushed coal burns intensely with the formation of a dazzling flame:
5C + 4KNO 3 = 2K 2 CO 3 + ZCO 2 + 2N 2
1. Formation of salt-like carbides with active metals.
A significant weakening of the non-metallic properties of carbon is expressed in the fact that its functions as an oxidizing agent are manifested to a much lesser extent than its reducing functions.
2. Only in reactions with active metals do carbon atoms transform into negatively charged ions C -4 and (C=C) 2-, forming salt-like carbides:
ZS + 4Al = Al 4 C 3 aluminum carbide
2C + Ca = CaC 2 calcium carbide
3. Carbides ionic type- very unstable compounds, they easily decompose under the influence of acids and water, which indicates the instability of negatively charged carbon anions:
Al 4 C 3 + 12H 2 O = ZSN 4 + 4Al(OH) 3
CaC 2 + 2H 2 O = C 2 H 2 + Ca(OH) 2
4. Formation of covalent compounds with metals
In melts of mixtures of carbon with transition metals carbides are formed mainly from covalent type communications. Their molecules have a variable composition, and the substances as a whole are close to alloys. Such carbides are highly stable; they are chemically inert with respect to water, acids, alkalis and many other reagents.
5. Interaction with hydrogen
At high T and P, in the presence of a nickel catalyst, carbon combines with hydrogen:
C + 2H 2 → CH 4
The reaction is highly reversible and has no practical significance.
Carbon(II) monoxide– CO
(carbon monoxide, carbon monoxide, carbon monoxide)
Physical properties: a colorless, poisonous gas, tasteless and odorless, burns with a bluish flame, lighter than air, poorly soluble in water. The concentration of carbon monoxide in the air is 12.5-74% explosive.
Receipt:
1) In industry
C + O 2 = CO 2 + 402 kJ
CO 2 + C = 2CO – 175 kJ
In gas generators, water vapor is sometimes blown through hot coal:
C + H 2 O = CO + H 2 – Q,
a mixture of CO + H 2 is called synthesis gas.
2) In the laboratory- thermal decomposition of formic or oxalic acid in the presence of H 2 SO 4 (conc.):
HCOOH t˚C, H2SO4 → H2O+CO
H2C2O4 t˚C,H2SO4 → CO + CO 2 + H 2 O
Chemical properties:
Under normal conditions, CO is inert; when heated - a reducing agent;
CO - non-salt-forming oxide.
1) with oxygen
2C +2 O + O 2 t ˚ C → 2C +4 O 2
2) with metal oxides CO + Me x O y = CO 2 + Me
C +2 O + CuO t ˚ C → Сu + C +4 O 2
3) with chlorine (in the light)
CO + Cl 2 light → COCl 2 (phosgene - poisonous gas)
4)* reacts with alkali melts (under pressure)
CO + NaOH P → HCOONa (sodium formate)
The effect of carbon monoxide on living organisms:
Carbon monoxide is dangerous because it prevents the blood from carrying oxygen to vital organs such as the heart and brain. Carbon monoxide combines with hemoglobin, which carries oxygen to the body's cells, making the body unsuitable for oxygen transport. Depending on the amount inhaled, carbon monoxide impairs coordination, aggravates cardiovascular diseases and causes fatigue, headaches, and weakness. The effect of carbon monoxide on human health depends on its concentration and the time of exposure to the body. A concentration of carbon monoxide in the air of more than 0.1% leads to death within one hour, and a concentration of more than 1.2% within three minutes.
Applications of carbon monoxide:
Carbon monoxide is mainly used as a flammable gas mixed with nitrogen, the so-called generator or air gas, or water gas mixed with hydrogen. In metallurgy for the recovery of metals from their ores. To obtain high purity metals from the decomposition of carbonyls.
Carbon monoxide (IV) CO2 – carbon dioxide
Physical properties: Carbon dioxide, colorless, odorless, solubility in water - 0.9V CO 2 dissolves in 1V H 2 O (at normal conditions); heavier than air; t°pl.= -78.5°C (solid CO 2 is called “dry ice”); does not support combustion.
Molecule structure:
Carbon dioxide has the following electronic and structural formula -
3. Combustion of carbon-containing substances:
CH 4 + 2O 2 → 2H2O + CO2
4. When slow oxidation in biochemical processes (respiration, decay, fermentation)
Chemical properties:
- Designation - C (Carbon);
- Period - II;
- Group - 14 (IVa);
- Atomic mass - 12.011;
- Atomic number - 6;
- Atomic radius = 77 pm;
- Covalent radius = 77 pm;
- Electron distribution - 1s 2 2s 2 2p 2 ;
- melting temperature = 3550°C;
- boiling point = 4827°C;
- Electronegativity (according to Pauling/according to Alpred and Rochow) = 2.55/2.50;
- Oxidation state: +4, +3, +2, +1, 0, -1, -2, -3, -4;
- Density (no.) = 2.25 g/cm 3 (graphite);
- Molar volume = 5.3 cm 3 /mol.
Carbon as charcoal known to man since time immemorial, therefore, it makes no sense to talk about the date of its discovery. Actually, “carbon” received its name in 1787, when the book “Method of chemical nomenclature", in which instead of French name“clean coal” (charbone pur) the term “carbon” (carbone) appeared.
Carbon has a unique ability to form polymer chains of unlimited length, thereby giving rise to a huge class of compounds, the study of which is dealt with in a separate branch of chemistry - organic chemistry. Organic compounds carbon is the basis of terrestrial life, therefore, about the importance of carbon, how chemical element, it makes no sense to say - it is the basis of life on Earth.
Now let's look at carbon from the point of view of inorganic chemistry.

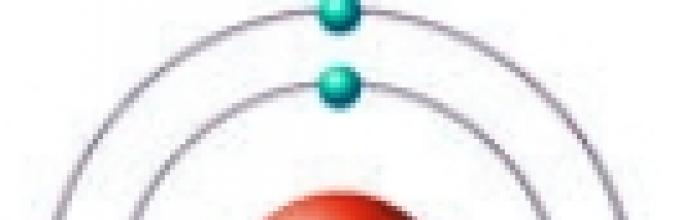
Rice. Structure of the carbon atom.
The electronic configuration of carbon is 1s 2 2s 2 2p 2 (see Electronic structure of atoms). On the outside energy level Carbon has 4 electrons: 2 paired in the s-sublevel + 2 unpaired in p-orbitals. When a carbon atom transitions to an excited state (requires energy expenditure), one electron from the s-sublevel “leaves” its pair and moves to the p-sublevel, where there is one free orbital. Thus, in an excited state electronic configuration carbon atom takes the following form: 1s 2 2s 1 2p 3.

Rice. The transition of a carbon atom to an excited state.
This “castling” significantly expands the valence capabilities of carbon atoms, which can take an oxidation state from +4 (in compounds with active non-metals) to -4 (in compounds with metals).
In an unexcited state, the carbon atom in compounds has a valency of 2, for example, CO(II), and in an excited state it has a valency of 4: CO 2 (IV).
The “uniqueness” of the carbon atom lies in the fact that at its outer energy level there are 4 electrons, therefore, to complete the level (which, in fact, the atoms of any chemical element strive for), it can, with equal “success,” both give and add electrons with the formation covalent bonds(See Covalent bond).
Carbon as a simple substance
As a simple substance, carbon can be found in the form of several allotropic modifications:
- Diamond
- Graphite
- Fullerene
- Carbin
Diamond

Rice. Crystal cell diamond
Properties of diamond:
- colorless crystalline substance;
- the most solid in nature;
- has a strong refractive effect;
- poorly conducts heat and electricity.

Rice. Diamond tetrahedron.
The exceptional hardness of diamond is explained by the structure of its crystal lattice, which has the shape of a tetrahedron - in the center of the tetrahedron there is a carbon atom, which is connected by equally strong bonds with four neighboring atoms that form the vertices of the tetrahedron (see figure above). This “construction”, in turn, is connected with neighboring tetrahedrons.
Graphite

Rice. Graphite crystal lattice.
Properties of graphite:
- soft crystalline substance of gray color with a layered structure;
- has a metallic luster;
- conducts electricity well.
In graphite, carbon atoms form regular hexagons lying in the same plane, organized into endless layers.
In graphite chemical bonds between neighboring carbon atoms are formed due to the three valence electrons of each atom (shown in blue in the figure below), while the fourth electron (shown in red) of each carbon atom, located in the p-orbital lying perpendicular to the plane of the graphite layer, does not participate in the formation covalent bonds in the plane of the layer. Its “purpose” is different - interacting with its “brother” lying in the adjacent layer, it provides a connection between the layers of graphite, and the high mobility of p-electrons determines the good electrical conductivity of graphite.

Rice. Distribution of carbon atom orbitals in graphite.
Fullerene

Rice. Crystal lattice of fullerene.
Fullerene properties:
- a fullerene molecule is a collection of carbon atoms closed in hollow spheres like a soccer ball;
- it is a fine-crystalline substance of yellow-orange color;
- melting point = 500-600°C;
- semiconductor;
- is part of the shungite mineral.
Carbin
Carbyne properties:
- black inert substance;
- consists of polymer linear molecules in which the atoms are connected by alternating single and triple bonds;
- semiconductor.
Chemical properties of carbon
Under normal conditions, carbon is an inert substance, but when heated it can react with a variety of simple and complex substances.
It was already said above that at the external energy level of carbon there are 4 electrons (neither here nor there), therefore carbon can both give electrons and accept them, manifesting itself in some compounds restorative properties, and in others - oxidative.
Carbon is reducing agent in reactions with oxygen and other elements having higher electronegativity (see table of electronegativity of elements):
- when heated in air it burns (with an excess of oxygen with the formation of carbon dioxide; with its deficiency - carbon monoxide (II)):
C + O 2 = CO 2;
2C + O 2 = 2CO. - reacts at high temperatures with sulfur vapor, easily interacts with chlorine, fluorine:
C + 2S = CS 2
C + 2Cl 2 = CCl 4
2F 2 + C = CF 4 - When heated, it reduces many metals and non-metals from oxides:
C0 + Cu +2 O = Cu 0 + C +2 O;
C 0 +C +4 O 2 = 2C +2 O - at a temperature of 1000°C it reacts with water (gasification process), forming water gas:
C + H 2 O = CO + H 2;
Carbon exhibits oxidizing properties in reactions with metals and hydrogen:
- reacts with metals to form carbides:
Ca + 2C = CaC 2 - interacting with hydrogen, carbon forms methane:
C + 2H 2 = CH 4
Carbon is obtained by thermal decomposition of its compounds or pyrolysis of methane (at high temperature):
CH 4 = C + 2H 2.
Application of carbon
Carbon compounds have found the widest application in national economy, it is not possible to list all of them, we will indicate only a few:
- graphite is used to make pencil leads, electrodes, melting crucibles, as a neutron moderator in nuclear reactors, and as a lubricant;
- Diamonds are used in jewelry, as a cutting tool, in drilling equipment, and as an abrasive material;
- Carbon is used as a reducing agent to produce some metals and non-metals (iron, silicon);
- carbon makes up the bulk of activated carbon, which has found wide application, both in everyday life (for example, as an adsorbent for purifying air and solutions), and in medicine (activated carbon tablets) and in industry (as a carrier for catalytic additives, a polymerization catalyst etc.).
Carbon monoxide (IV), carbonic acid and their salts
Comprehensive purpose of the module: know methods for producing carbon (IV) oxide and hydroxide; describe them physical properties; know the characteristics of acid-base properties; characterize redox properties.
All elements of the carbon subgroup form oxides with the general formula EO 2. CO 2 and SiO 2 exhibit acid properties, GeО 2 , SnО 2 , PbО 2 exhibit amphoteric properties with a predominance of acidic ones, and in the subgroup from top to bottom the acidic properties weaken.
The oxidation state (+4) for carbon and silicon is very stable, so the oxidizing properties of the compound are very difficult to exhibit. In the germanium subgroup, the oxidizing properties of compounds (+4) increase due to destabilization highest degree oxidation.
Carbon monoxide (IV), carbonic acid and their salts
Carbon dioxide CO 2 (carbon dioxide) - under normal conditions it is a colorless and odorless gas, slightly sour taste, about 1.5 times heavier than air, soluble in water, liquefied quite easily - at room temperature it can be turned into liquid under a pressure of about 60 10 5 Pa. When cooled to? 56.2°C, liquid carbon dioxide solidifies and turns into a snow-like mass.
In all states of aggregation consists of non-polar linear molecules. Chemical structure CO 2 is determined by sp-hybridization of the central carbon atom and the formation of additional p-p bonds: O = C = O
Some part of the CO 2 dissolved in the will interacts with it by forming carbonic acid
CO 2 + H 2 O - CO 2 H 2 O - H 2 CO 3.
Carbon dioxide is very easily absorbed by alkali solutions to form carbonates and bicarbonates:
CO 2 + 2NaOH = Na 2 CO 3 + H 2 O;
CO 2 + NaOH = NaHCO 3.
CO 2 molecules are very thermally stable; decomposition begins only at a temperature of 2000°C. Therefore, carbon dioxide does not burn and does not support the combustion of conventional fuel. But in its atmosphere some are burning simple substances, the atoms of which exhibit a high affinity for oxygen, for example, magnesium ignites when heated in a CO 2 atmosphere.
Carbonic acid and its salts
Carbonic acid H 2 CO 3 is a weak compound and exists only in aqueous solutions. Most of the carbon dioxide dissolved in water is in the form of hydrated CO 2 molecules, a smaller part forms carbonic acid.
Aqueous solutions in equilibrium with atmospheric CO2 are acidic: = 0.04 M and pH? 4.
Carbonic acid is dibasic, belongs to weak electrolytes, dissociates stepwise (K1 = 4.4 10?7; K2 = 4.8 10?11). When CO 2 is dissolved in water, the following dynamic equilibrium is established:
H 2 O + CO 2 - CO 2 H 2 O - H 2 CO 3 - H + + HCO 3 ?
When heated aqueous solution carbon dioxide, the solubility of the gas decreases, CO 2 is released from the solution, and the equilibrium shifts to the left.
Carbonic acid salts
Being dibasic, carbonic acid forms two series of salts: medium salts (carbonates) and acidic salts (bicarbonates). Most carbonic acid salts are colorless. Of the carbonates, only alkali metal and ammonium salts are soluble in water.
In water, carbonates undergo hydrolysis, and therefore their solutions have an alkaline reaction:
Na 2 CO 3 + H 2 O - NaHCO 3 + NaOH.
Further hydrolysis with the formation of carbonic acid practically does not occur under normal conditions.
The dissolution of hydrocarbonates in water is also accompanied by hydrolysis, but to a much lesser extent, and the environment is created slightly alkaline (pH 8).
Ammonium carbonate (NH 4) 2 CO 3 is highly volatile at elevated and even normal temperatures, especially in the presence of water vapor, which causes severe hydrolysis
Strong acids and even weak acetic acid displace carbonic acid from carbonates:
K 2 CO 3 + H 2 SO 4 = K 2 SO 4 + H 2 O + CO 2 ^.
Unlike most carbonates, all bicarbonates are soluble in water. They are less stable than carbonates of the same metals and, when heated, easily decompose, turning into the corresponding carbonates:
2KHCO 3 = K 2 CO 3 + H 2 O + CO 2 ^;
Ca(HCO 3) 2 = CaCO 3 + H 2 O + CO 2 ^.
Strong acids hydrocarbonates decompose like carbonates:
KHCO 3 + H 2 SO 4 = KHSO 4 + H 2 O + CO 2
From salts of carbonic acid highest value have: sodium carbonate (soda), potassium carbonate (potash), calcium carbonate (chalk, marble, limestone), sodium bicarbonate (baking soda) and basic copper carbonate (CuOH) 2 CO 3 (malachite).
Basic salts of carbonic acid are practically insoluble in water and easily decompose when heated:
(CuOH) 2 CO 3 = 2CuO + CO 2 + H 2 O.
In general, the thermal stability of carbonates depends on the polarization properties of the ions that make up the carbonate. The more polarizing the cation has on the carbonate ion, the lower the decomposition temperature of the salt. If the cation can be easily deformed, then the carbonate ion itself will also have a polarizing effect on the cation, which will lead to a sharp decrease in the decomposition temperature of the salt.
Sodium and potassium carbonates melt without decomposition, and most other carbonates decompose into metal oxide and carbon dioxide when heated.
(IV) (CO 2, carbon dioxide, carbon dioxide) is a colorless, tasteless and odorless gas that is heavier than air and soluble in water.
Under normal conditions, solid carbon dioxide passes directly into a gaseous state, bypassing the liquid state.
At large quantities carbon monoxide, people begin to suffocate. Concentrations of more than 3% lead to rapid breathing, and above 10% there is loss of consciousness and death.
Chemical properties of carbon monoxide.
Carbon monoxide - it is carbonic anhydride H 2 CO 3 .
If carbon monoxide is passed through calcium hydroxide (limewater), a white precipitate forms:
Ca(OH) 2 + CO 2 = CaCO 3 ↓ + H 2 O
If carbon dioxide is taken in excess, then the formation of bicarbonates is observed, which dissolve in water:
CaCO 3 + H 2 O + CO 2 = Ca(HCO 3) 2,
Which then disintegrate when heated:
2KNCO 3 = K 2 CO 3 + H 2 O + CO 2
Application of carbon monoxide.
Carbon dioxide is used in various industries. IN chemical production- as a refrigerant.
IN Food Industry it is used as a preservative E290. Although he was assigned “conditionally safe”, in reality this is not the case. Doctors have proven that frequent consumption of E290 leads to the accumulation of a toxic toxic compound. Therefore, you need to read product labels more carefully.