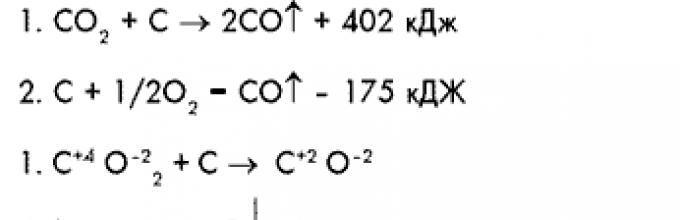Oxides of carbon (II) and (IV)
Integrated lesson in chemistry and biology
Tasks: study and systematize knowledge about carbon oxides (II) and (IV); reveal the relationship between living and inanimate nature; consolidate knowledge about the effect of carbon oxides on the human body; strengthen your skills in working with laboratory equipment.
Equipment: HCl solution, litmus, Ca(OH) 2, CaCO 3, glass rod, homemade tables, portable board, ball-and-stick model.
PROGRESS OF THE LESSON
Biology teacher communicates the topic and objectives of the lesson.
Chemistry teacher. Based on the doctrine of covalent bonds, compose the electronic and structural formulas of carbon oxides (II) and (IV).
The chemical formula of carbon monoxide (II) is CO, the carbon atom is in its normal state.
Due to the pairing of unpaired electrons, two polar covalent bonds are formed, and the third covalent bond is formed by the donor-acceptor mechanism. The donor is an oxygen atom, because it provides a free pair of electrons; the acceptor is a carbon atom, because provides an empty orbital.
In industry, carbon (II) monoxide is produced by passing CO 2 over hot coal at high temperature. It is also formed during the combustion of coal with a lack of oxygen. ( A student writes the reaction equation on the board)

In the laboratory, CO is produced by the action of concentrated H 2 SO 4 on formic acid. ( The teacher writes down the reaction equation.)
![]()
Biology teacher. So, you have become acquainted with the production of carbon monoxide (II). What physical properties does carbon monoxide (II) have?
Student. It is a colorless gas, poisonous, odorless, lighter than air, poorly soluble in water, boiling point –191.5 °C, solidifies at –205 °C.
Chemistry teacher. Carbon monoxide in quantities dangerous to human life, found in car exhaust gases. Therefore, garages should be well ventilated, especially when starting the engine.
Biology teacher. What effect does carbon monoxide have on the human body?
Student. Carbon monoxide is extremely toxic to humans - this is explained by the fact that it forms carboxyhemoglobin. Carboxyhemoglobin is a very strong compound. As a result of its formation, blood hemoglobin does not interact with oxygen, and in case of severe poisoning, a person can die from oxygen starvation.
Biology teacher. What first aid should a person receive for carbon monoxide poisoning?
Students. It is necessary to call an ambulance, the victim must be taken outside, artificial respiration must be performed, and the room must be well ventilated.
Chemistry teacher. Write the chemical formula of carbon monoxide (IV) and, using the ball-and-stick model, construct its structure.
The carbon atom is in an excited state. All four polar covalent bonds were formed by pairing unpaired electrons. However, due to its linear structure, its molecule as a whole is non-polar.
In industry, CO 2 is obtained from the decomposition of calcium carbonate in the production of lime.
(A student writes down the reaction equation.)
In the laboratory, CO 2 is obtained by reacting acids with chalk or marble.
(Students perform a laboratory experiment.)

Biology teacher. What processes result in the formation of carbon dioxide in the body?
Student. Carbon dioxide is formed in the body as a result of oxidation reactions organic matter included in the cell.
(Students perform a laboratory experiment.)

The lime mortar became cloudy because calcium carbonate is formed. In addition to the respiration process, CO2 is released as a result of fermentation and decay.
Biology teacher. Does physical activity affect the breathing process?
Student. With excessive physical (muscular) stress, the muscles use oxygen faster than the blood can deliver it, and then they synthesize the ATP necessary for their work through fermentation. Lactic acid C 3 H 6 O 3 is formed in the muscles, which enters the blood. The accumulation of large amounts of lactic acid is harmful to the body. After heavy physical activity, we continue to breathe heavily for some time - we pay off the “oxygen debt”.
Chemistry teacher. Large quantity Carbon (IV) monoxide is released into the atmosphere when fossil fuels are burned. At home, we use natural gas as fuel, and it consists of almost 90% methane (CH 4). I invite one of you to go to the board, write an equation for the reaction and analyze it from the point of view of oxidation-reduction.

Biology teacher. Why can't you use gas stoves to heat a room?
Student. Methane – component natural gas. When it burns, the carbon dioxide content in the air increases and the oxygen content decreases. ( Working with the Table of Contents CO 2 in the air".)
When the air contains 0.3% CO 2, a person experiences rapid breathing; at 10% - loss of consciousness, at 20% - instant paralysis and quick death. A child especially needs clean air, because the oxygen consumption of the tissues of a growing body is greater than that of an adult. Therefore, it is necessary to regularly ventilate the room. If there is excess CO 2 in the blood, the excitability of the respiratory center increases and breathing becomes more frequent and deeper.
Biology teacher. Let's consider the role of carbon monoxide (IV) in plant life.
Student. In plants, the formation of organic substances occurs from CO 2 and H 2 O in the light; in addition to organic substances, oxygen is formed.

![]()
Photosynthesis regulates the amount of carbon dioxide in the atmosphere, which prevents the planet's temperature from rising. Every year, plants absorb 300 billion tons of carbon dioxide from the atmosphere. The process of photosynthesis releases 200 billion tons of oxygen into the atmosphere annually. Ozone is formed from oxygen during a thunderstorm.
Chemistry teacher. Let's consider chemical properties carbon monoxide (IV).

Biology teacher. What is the importance of carbonic acid in the human body during respiration? ( Filmstrip fragment.)
Enzymes in the blood convert carbon dioxide into carbonic acid, which dissociates into hydrogen and bicarbonate ions. If the blood contains an excess of H + ions, i.e. if the acidity of the blood is increased, then some of the H + ions combine with bicarbonate ions, forming carbonic acid and thereby freeing the blood from excess H + ions. If there are too few H + ions in the blood, then carbonic acid dissociates and the concentration of H + ions in the blood increases. At a temperature of 37 °C, the blood pH is 7.36.
In the body, carbon dioxide is transported by the blood in the form of chemical compounds - sodium and potassium bicarbonates.
Fixing the material
Test
From the proposed gas exchange processes in the lungs and tissues, those completing the first option must choose the codes of the correct answers on the left, and the second - on the right.
(1) Transition of O 2 from the lungs to the blood. (13)
(2) Transfer of O 2 from blood to tissues. (14)
(3) Transition of CO 2 from tissues to blood. (15)
(4) Transition of CO 2 from the blood to the lungs. (16)
(5) O2 absorption by red blood cells. (17)
(6) Release of O 2 from red blood cells. (18)
(7) Conversion of arterial blood into venous blood. (19)
(8) Conversion of venous blood into arterial blood. (20)
(9) Break chemical bond O 2 with hemoglobin. (21)
(10) Chemical binding of O 2 to hemoglobin. (22)
(11) Capillaries in tissues. (23)
(12) Pulmonary capillaries. (24)
First option questions
1. Gas exchange processes in tissues.
2. Physical processes during gas exchange.
Second option questions
1.
Gas exchange processes in the lungs.
2. Chemical processes during gas exchange
Task
Determine the volume of carbon monoxide (IV) that is released during the decomposition of 50 g of calcium carbonate.
Carbon
In the free state, carbon forms 3 allotropic modifications: diamond, graphite and artificially produced carbyne.
In a diamond crystal, each carbon atom is connected by strong covalent bonds to four others placed around it at equal distances.
All carbon atoms are in a state of sp 3 hybridization. The atomic crystal lattice of diamond has a tetrahedral structure.
Diamond is a colorless, transparent, highly refracting substance. It has the greatest hardness among all known substances. Diamond is brittle, refractory, conducts heat poorly and electric current. The small distances between neighboring carbon atoms (0.154 nm) determine the rather high density of diamond (3.5 g/cm3).
IN crystal lattice of graphite, each carbon atom is in a state of sp 2 hybridization and forms three strong covalent bonds with carbon atoms located in the same layer. Three electrons of each carbon atom participate in the formation of these bonds, and the fourth valence electrons form n-bonds and are relatively free (mobile). They determine the electrical and thermal conductivity of graphite.
The length of the covalent bond between neighboring carbon atoms in the same plane is 0.152 nm, and the distance between C atoms in different layers is 2.5 times greater, so the bonds between them are weak.
Graphite is an opaque, soft, greasy to the touch substance of gray-black color with a metallic sheen; conducts heat and electricity well. Graphite has a lower density compared to diamond and easily splits into thin flakes.
The disordered structure of fine-crystalline graphite underlies the structure various forms amorphous carbon, the most important of which are coke, brown and hard coals, soot, activated (active) carbon.
This allotropic modification of carbon is obtained by catalytic oxidation (dehydropolycondensation) of acetylene. Carbyne is a chain polymer that has two forms:
С=С-С=С-... and...=С=С=С=
Carbyne has semiconducting properties.
At ordinary temperatures, both modifications of carbon (diamond and graphite) are chemically inert. Fine-crystalline forms of graphite - coke, soot, activated carbon - are more reactive, but, as a rule, after they are preheated to a high temperature.
1. Interaction with oxygen
C + O 2 = CO 2 + 393.5 kJ (in excess O 2)
2C + O 2 = 2CO + 221 kJ (with a lack of O 2)
Coal combustion is one of the most important sources of energy.
2. Interaction with fluorine and sulfur.
C + 2F 2 = CF 4 carbon tetrafluoride
C + 2S = CS 2 carbon disulfide
3. Coke is one of the most important reducing agents used in industry. In metallurgy, it is used to obtain metals from oxides, for example:
ZS + Fe 2 O 3 = 2Fe + ZSO
C + ZnO = Zn + CO
4. When carbon interacts with alkaline and alkaline earth metals the reduced metal combines with carbon to form a carbide. For example: 3S + CaO = CaC 2 + CO calcium carbide
5. Coke is also used to produce silicon:
2C + SiO 2 = Si + 2СО
6. If there is an excess of coke, silicon carbide (carborundum) SiC is formed.
Production of “water gas” (gasification of solid fuel)
By passing water vapor through hot coal, a flammable mixture of CO and H 2, called water gas, is obtained:
C + H 2 O = CO + H 2
7. Reactions with oxidizing acids.
Activated or charcoal when heated, it reduces the anions NO 3 - and SO 4 2- from concentrated acids:
C + 4HNO 3 = CO 2 + 4NO 2 + 2H 2 O
C + 2H 2 SO 4 = CO 2 + 2SO 2 + 2H 2 O
8. Reactions with molten nitrates alkali metals
In KNO 3 and NaNO 3 melts, crushed coal burns intensely with the formation of a dazzling flame:
5C + 4KNO 3 = 2K 2 CO 3 + ZCO 2 + 2N 2
1. Formation of salt-like carbides with active metals.
A significant weakening of the non-metallic properties of carbon is expressed in the fact that its functions as an oxidizing agent are manifested to a much lesser extent than its reducing functions.
2. Only in reactions with active metals do carbon atoms transform into negatively charged ions C -4 and (C=C) 2-, forming salt-like carbides:
ZS + 4Al = Al 4 C 3 aluminum carbide
2C + Ca = CaC 2 calcium carbide
3. Carbides ionic type- very unstable compounds, they easily decompose under the influence of acids and water, which indicates the instability of negatively charged carbon anions:
Al 4 C 3 + 12H 2 O = ZSN 4 + 4Al(OH) 3
CaC 2 + 2H 2 O = C 2 H 2 + Ca(OH) 2
4. Formation of covalent compounds with metals
In melts of mixtures of carbon with transition metals carbides are formed mainly from covalent type communications. Their molecules have a variable composition, and the substances as a whole are close to alloys. Such carbides are highly stable; they are chemically inert with respect to water, acids, alkalis and many other reagents.
5. Interaction with hydrogen
At high T and P, in the presence of a nickel catalyst, carbon combines with hydrogen:
C + 2H 2 → CH 4
The reaction is highly reversible and has no practical significance.
Carbon(II) monoxide– CO
(carbon monoxide, carbon monoxide, carbon monoxide)
Physical properties: a colorless, poisonous gas, tasteless and odorless, burns with a bluish flame, lighter than air, poorly soluble in water. The concentration of carbon monoxide in the air is 12.5-74% explosive.
Receipt:
1) In industry
C + O 2 = CO 2 + 402 kJ
CO 2 + C = 2CO – 175 kJ
In gas generators, water vapor is sometimes blown through hot coal:
C + H 2 O = CO + H 2 – Q,
a mixture of CO + H 2 is called synthesis gas.
2) In the laboratory- thermal decomposition of formic or oxalic acid in the presence of H 2 SO 4 (conc.):
HCOOH t˚C, H2SO4 → H2O+CO
H2C2O4 t˚C,H2SO4 → CO + CO 2 + H 2 O
Chemical properties:
Under normal conditions, CO is inert; when heated - a reducing agent;
CO - non-salt-forming oxide.
1) with oxygen
2C +2 O + O 2 t ˚ C → 2C +4 O 2
2) with metal oxides CO + Me x O y = CO 2 + Me
C +2 O + CuO t ˚ C → Сu + C +4 O 2
3) with chlorine (in the light)
CO + Cl 2 light → COCl 2 (phosgene - poisonous gas)
4)* reacts with alkali melts (under pressure)
CO + NaOH P → HCOONa (sodium formate)
The effect of carbon monoxide on living organisms:
Carbon monoxide is dangerous because it prevents the blood from carrying oxygen to vital organs such as the heart and brain. Carbon monoxide combines with hemoglobin, which carries oxygen to the body's cells, making the body unsuitable for oxygen transport. Depending on the amount inhaled, carbon monoxide impairs coordination, aggravates cardiovascular diseases and causes fatigue, headaches, and weakness. The effect of carbon monoxide on human health depends on its concentration and the time of exposure to the body. A concentration of carbon monoxide in the air of more than 0.1% leads to death within one hour, and a concentration of more than 1.2% within three minutes.
Applications of carbon monoxide:
Carbon monoxide is mainly used as a flammable gas mixed with nitrogen, the so-called generator or air gas, or water gas mixed with hydrogen. In metallurgy for the recovery of metals from their ores. To obtain high purity metals from the decomposition of carbonyls.
Carbon monoxide (IV) CO2 – carbon dioxide
Physical properties: Carbon dioxide, colorless, odorless, solubility in water - 0.9V CO 2 dissolves in 1V H 2 O (at normal conditions); heavier than air; t°pl. = -78.5°C (solid CO 2 is called “dry ice”); does not support combustion.
Molecule structure:
Carbon dioxide has the following electronic and structural formulas -
3. Combustion of carbon-containing substances:
CH 4 + 2O 2 → 2H2O + CO2
4. With slow oxidation in biochemical processes (respiration, rotting, fermentation)
Chemical properties:
Carbon dioxide, also known as 4, reacts with a number of substances, forming compounds that vary in composition and chemical properties. Consisting of nonpolar molecules, it has very weak intermolecular bonds and can only be present if the temperature is higher than 31 degrees Celsius. Carbon dioxide is a chemical compound consisting of one carbon atom and two oxygen atoms.
Carbon monoxide 4: formula and basic information
Carbon dioxide is present in low concentrations in the Earth's atmosphere and acts as a greenhouse gas. His chemical formula CO2. At high temperatures it can exist exclusively in a gaseous state. In its solid state, it is called dry ice.
Carbon dioxide is an important component of the carbon cycle. It comes from a variety of natural sources, including volcanic degassing, combustion of organic matter, and the respiratory processes of living aerobic organisms. Anthropogenic sources of carbon dioxide mainly come from the combustion of various fossil fuels for electricity generation and transportation.

It is also produced by various microorganisms from fermentation and cellular respiration. Plants convert carbon dioxide into oxygen during a process called photosynthesis, using both carbon and oxygen to form carbohydrates. In addition, plants also release oxygen into the atmosphere, which is then used for respiration by heterotrophic organisms.
Carbon dioxide (CO2) in the body
Carbon monoxide 4 reacts with different substances and is a gaseous waste product from metabolism. There is more than 90% of it in the blood in the form of bicarbonate (HCO 3). The rest is either dissolved CO 2 or carbonic acid (H2CO 3). Organs such as the liver and kidneys are responsible for balancing these compounds in the blood. Bicarbonate is chemical substance, which acts as a buffer. It keeps the blood pH level at the required level, avoiding an increase in acidity.

Structure and properties of carbon dioxide
Carbon dioxide (CO2) is a chemical compound that is a gas when room temperature and above. It consists of one carbon atom and two oxygen atoms. Humans and animals release carbon dioxide when they exhale. In addition, it is formed whenever something organic is burned. Plants use carbon dioxide to produce food. This process is called photosynthesis.
The properties of carbon dioxide were studied by Scottish scientist Joseph Black back in the 1750s. capable of capturing thermal energy and influencing the climate and weather on our planet. It is the cause of global warming and an increase in the temperature of the Earth's surface.

Biological role
Carbon monoxide 4 reacts with various substances and is the final product in organisms that obtain energy from the breakdown of sugars, fats and amino acids. This process is known to be characteristic of all plants, animals, many fungi and some bacteria. In higher animals, carbon dioxide moves in the blood from body tissues to the lungs, where it is exhaled. Plants obtain it from the atmosphere for use in photosynthesis.
Dry ice
Dry ice or solid carbon dioxide is the solid state of CO 2 gas with a temperature of -78.5 °C. This substance does not occur naturally in nature, but is produced by humans. It is colorless and can be used in the preparation of carbonated drinks, as a cooling element in ice cream containers and in cosmetology, for example for freezing warts. Dry ice vapor is suffocating and can cause death. Use caution and professionalism when using dry ice.
Under normal pressure it will not melt from a liquid, but instead goes directly from a solid to a gas. This is called sublimation. It will change directly from solid to gas at any temperature exceeding extremely low temperatures. Dry ice sublimates at normal air temperatures. This releases carbon dioxide, which is odorless and colorless. Carbon dioxide can be liquefied at pressures above 5.1 atm. The gas that comes from dry ice is so cold that when mixed with air, it cools the water vapor in the air into a mist that looks like thick white smoke.

Preparation, chemical properties and reactions
In industry, carbon monoxide 4 is produced in two ways:
- By burning fuel (C + O 2 = CO 2).
- By thermal decomposition of limestone (CaCO 3 = CaO + CO 2).
The resulting volume of carbon monoxide 4 is purified, liquefied and pumped into special cylinders.
Being acidic, carbon monoxide 4 reacts with substances such as:
- Water. When dissolved, carbonic acid (H 2 CO 3) is formed.
- Alkaline solutions. Carbon monoxide 4 (formula CO 2) reacts with alkalis. In this case, medium and acidic salts (NaHCO 3) are formed.
- These reactions produce carbonate salts (CaCO 3 and Na 2 CO 3).
- Carbon. When carbon monoxide 4 reacts with hot coal, carbon monoxide 2 (carbon monoxide) is formed, which can cause poisoning. (CO 2 + C = 2CO).
- Magnesium. As a rule, carbon dioxide does not support combustion; only at very high temperatures can it react with certain metals. For example, ignited magnesium will continue to burn in CO 2 during a redox reaction (2Mg + CO 2 = 2MgO + C).

The qualitative reaction of carbon monoxide 4 manifests itself when passing it through limestone water (Ca(OH) 2 or through barite water (Ba(OH) 2). Turbidity and precipitation can be observed. If you continue to pass carbon dioxide after this, the water will become clear again , since insoluble carbonates are converted into soluble bicarbonates (acid salts of carbonic acid).

Carbon dioxide is also produced by the combustion of all carbon-containing fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), coal or wood. In most cases, water is also released.
Carbon dioxide (carbon dioxide) is made up of one carbon atom and two oxygen atoms, which are held together by covalent bonds (or sharing of electrons). Pure carbon is very rare. It occurs in nature only in the form of minerals, graphite and diamond. Despite this, it is a building block of life that, when combined with hydrogen and oxygen, forms the basic compounds that make up everything on the planet.

Hydrocarbons such as coal, oil and natural gas are compounds made of hydrogen and carbon. This element is found in calcite (CaCo 3), minerals in sedimentary and metamorphic rocks, limestone and marble. It is the element that contains all organic matter - from fossil fuels to DNA.
Carbon (C)– typical non-metal; V periodic table is in the 2nd period of group IV, the main subgroup. Serial number 6, Ar = 12.011 amu, nuclear charge +6.Physical properties: carbon forms many allotropic modifications: diamond- one of the most solids, graphite, coal, soot.
A carbon atom has 6 electrons: 1s 2 2s 2 2p 2 . The last two electrons are located in separate p-orbitals and are unpaired. In principle, this pair could occupy the same orbital, but in this case the interelectron repulsion greatly increases. For this reason, one of them takes 2p x, and the other, either 2p y , or 2p z orbitals.
The difference in the energy of the s- and p-sublevels of the outer layer is small, so the atom quite easily goes into an excited state, in which one of the two electrons from the 2s orbital passes to a free one 2 rub. A valence state appears with the configuration 1s 2 2s 1 2p x 1 2p y 1 2p z 1 . It is this state of the carbon atom that is characteristic of the diamond lattice—tetrahedral spatial arrangement of hybrid orbitals, identical length and energy of bonds.
This phenomenon is known to be called sp 3 -hybridization, and the emerging functions are sp 3 -hybrid . The formation of four sp 3 bonds provides the carbon atom with a more stable state than three r-r- and one s-s-connection. In addition to sp 3 hybridization, sp 2 and sp hybridization is also observed at the carbon atom . In the first case, mutual overlap occurs s- and two p-orbitals. Three equivalent sp 2 hybrid orbitals are formed, located in the same plane at an angle of 120° to each other. The third orbital p is unchanged and directed perpendicular to the plane sp2.

During sp hybridization, the s and p orbitals overlap. An angle of 180° arises between the two equivalent hybrid orbitals that are formed, while the two p-orbitals of each atom remain unchanged.

Allotropy of carbon. Diamond and graphite
In a graphite crystal, carbon atoms are located in parallel planes, occupying the vertices of regular hexagons. Each carbon atom is connected to three neighboring sp 2 hybrid bonds. The connection between parallel planes is carried out due to van der Waals forces. The free p-orbitals of each atom are directed perpendicular to the planes of covalent bonds. Their overlap explains the additional π bond between the carbon atoms. Thus, from the valence state in which the carbon atoms in a substance are located determines the properties of this substance.
Chemical properties of carbon
Most characteristic degrees oxidation: +4, +2.
At low temperatures carbon is inert, but when heated its activity increases.
Carbon as a reducing agent:
- with oxygen
C 0 + O 2 – t° = CO 2 carbon dioxide
with a lack of oxygen - incomplete combustion:
2C 0 + O 2 – t° = 2C +2 O carbon monoxide
- with fluorine
C + 2F 2 = CF 4
- with water vapor
C 0 + H 2 O – 1200° = C +2 O + H 2 water gas
- with metal oxides. This is how metal is smelted from ore.
C 0 + 2CuO – t° = 2Cu + C +4 O 2
- with acids - oxidizing agents:
C 0 + 2H 2 SO 4 (conc.) = C +4 O 2 + 2SO 2 + 2H 2 O
C 0 + 4HNO 3 (conc.) = C +4 O 2 + 4NO 2 + 2H 2 O
- forms carbon disulfide with sulfur:
C + 2S 2 = CS 2.
Carbon as an oxidizing agent:
- forms carbides with some metals
4Al + 3C 0 = Al 4 C 3
Ca + 2C 0 = CaC 2 -4
- with hydrogen - methane (as well as a huge amount organic compounds)
C0 + 2H2 = CH4
— with silicon, forms carborundum (at 2000 °C in an electric furnace):
Finding carbon in nature
Free carbon occurs in the form of diamond and graphite. In the form of compounds, carbon is found in minerals: chalk, marble, limestone - CaCO 3, dolomite - MgCO 3 *CaCO 3; hydrocarbonates - Mg(HCO 3) 2 and Ca(HCO 3) 2, CO 2 is part of the air; carbon is the main integral part natural organic compounds - gas, oil, coal, peat, is part of organic substances, proteins, fats, carbohydrates, amino acids that make up living organisms.

Inorganic carbon compounds
Neither C 4+ nor C 4- ions are formed during any conventional chemical processes: carbon compounds contain covalent bonds of different polarities.
Carbon monoxide CO
Carbon monoxide; colorless, odorless, slightly soluble in water, soluble in organic solvents, toxic, boiling point = -192°C; t pl. = -205°C.
Receipt
1) In industry (in gas generators):
C + O 2 = CO 2
2) In the laboratory - thermal decomposition of formic or oxalic acid in the presence of H 2 SO 4 (conc.):
HCOOH = H2O + CO
H 2 C 2 O 4 = CO + CO 2 + H 2 O
Chemical properties
Under normal conditions, CO is inert; when heated - a reducing agent; non-salt-forming oxide.
1) with oxygen
2C +2 O + O 2 = 2C +4 O 2
2) with metal oxides
C +2 O + CuO = Cu + C +4 O 2
3) with chlorine (in the light)
CO + Cl 2 – hn = COCl 2 (phosgene)
4) reacts with alkali melts (under pressure)
CO + NaOH = HCOONa (sodium formate)
5) forms carbonyls with transition metals
Ni + 4CO – t° = Ni(CO) 4
Fe + 5CO – t° = Fe(CO) 5
Carbon monoxide (IV) CO2
Carbon dioxide, colorless, odorless, solubility in water - 0.9V CO 2 dissolves in 1V H 2 O (under normal conditions); heavier than air; t°pl. = -78.5°C (solid CO 2 is called “dry ice”); does not support combustion.
Receipt
- Thermal decomposition of carbonic acid salts (carbonates). Limestone firing:
CaCO 3 – t° = CaO + CO 2
- Action strong acids for carbonates and bicarbonates:
CaCO 3 + 2HCl = CaCl 2 + H 2 O + CO 2
NaHCO 3 + HCl = NaCl + H 2 O + CO 2
ChemicalpropertiesCO2
Acid oxide: Reacts with basic oxides and bases to form carbonic acid salts
Na 2 O + CO 2 = Na 2 CO 3
2NaOH + CO 2 = Na 2 CO 3 + H 2 O
NaOH + CO 2 = NaHCO 3
At elevated temperatures may exhibit oxidizing properties
C +4 O 2 + 2Mg – t° = 2Mg +2 O + C 0
Qualitative reaction
Cloudiness of lime water:
Ca(OH) 2 + CO 2 = CaCO 3 ¯ (white precipitate) + H 2 O
It disappears when CO 2 is passed through lime water for a long time, because insoluble calcium carbonate turns into soluble bicarbonate:
CaCO 3 + H 2 O + CO 2 = Ca(HCO 3) 2
Carbonic acid and itssalt
H 2CO 3 - A weak acid, it exists only in aqueous solution:
CO 2 + H 2 O ↔ H 2 CO 3
Dibasic:
H 2 CO 3 ↔ H + + HCO 3 — Acid salts- bicarbonates, hydrocarbonates
HCO 3 - ↔ H + + CO 3 2- Medium salts - carbonates
All properties of acids are characteristic.
Carbonates and bicarbonates can transform into each other:
2NaHCO 3 – t° = Na 2 CO 3 + H 2 O + CO 2
Na 2 CO 3 + H 2 O + CO 2 = 2NaHCO 3
Metal carbonates (except alkali metals) decarboxylate when heated to form an oxide:
CuCO 3 – t° = CuO + CO 2
Qualitative reaction- “boiling” under the influence of a strong acid:
Na 2 CO 3 + 2HCl = 2NaCl + H 2 O + CO 2
CO 3 2- + 2H + = H 2 O + CO 2
Carbides
Calcium carbide:
CaO + 3 C = CaC 2 + CO
CaC 2 + 2 H 2 O = Ca(OH) 2 + C 2 H 2.
Acetylene is released when zinc, cadmium, lanthanum and cerium carbides react with water:
2 LaC 2 + 6 H 2 O = 2La(OH) 3 + 2 C 2 H 2 + H 2.
Be 2 C and Al 4 C 3 decompose with water to form methane:
Al 4 C 3 + 12 H 2 O = 4 Al(OH) 3 = 3 CH 4.
In technology, titanium carbides TiC, tungsten W 2 C (hard alloys), silicon SiC (carborundum - as an abrasive and material for heaters) are used.
Cyanide
obtained by heating soda in an atmosphere of ammonia and carbon monoxide:
Na 2 CO 3 + 2 NH 3 + 3 CO = 2 NaCN + 2 H 2 O + H 2 + 2 CO 2
Hydrocyanic acid HCN is an important product of the chemical industry and is widely used in organic synthesis. Its global production reaches 200 thousand tons per year. Electronic structure cyanide anion is similar to carbon monoxide (II), such particles are called isoelectronic:
C = O: [:C = N:] –
Cyanides (0.1-0.2% aqueous solution) used in gold mining:
2 Au + 4 KCN + H 2 O + 0.5 O 2 = 2 K + 2 KOH.
When boiling solutions of cyanide with sulfur or melting solids, they form thiocyanates:
KCN + S = KSCN.
When cyanides of low-active metals are heated, cyanide is obtained: Hg(CN) 2 = Hg + (CN) 2. Cyanide solutions are oxidized to cyanates:
2 KCN + O 2 = 2 KOCN.
Cyanic acid exists in two forms:
H-N=C=O; H-O-C = N:
In 1828, Friedrich Wöhler (1800-1882) obtained urea from ammonium cyanate: NH 4 OCN = CO(NH 2) 2 by evaporating an aqueous solution.
This event is usually regarded as the victory of synthetic chemistry over "vitalistic theory".
There is an isomer of cyanic acid - explosive acid
H-O-N=C.
Its salts (mercuric fulminate Hg(ONC) 2) are used in impact igniters.
Synthesis urea(urea):
CO 2 + 2 NH 3 = CO(NH 2) 2 + H 2 O. At 130 0 C and 100 atm.
Urea is a carbonic acid amide; there is also its “nitrogen analogue” – guanidine.
Carbonates
The most important inorganic compounds carbon – salts of carbonic acid (carbonates). H 2 CO 3 is a weak acid (K 1 = 1.3 10 -4; K 2 = 5 10 -11). Carbonate buffer supports carbon dioxide balance in the atmosphere. The world's oceans have a huge buffer capacity because they are open system. The main buffer reaction is the equilibrium during the dissociation of carbonic acid:
H 2 CO 3 ↔ H + + HCO 3 - .
When acidity decreases, additional absorption of carbon dioxide from the atmosphere occurs with the formation of acid:
CO 2 + H 2 O ↔ H 2 CO 3 .
As acidity increases, carbonate rocks (shells, chalk and limestone sediments in the ocean) dissolve; this compensates for the loss of hydrocarbonate ions:
H + + CO 3 2- ↔ HCO 3 —
CaCO 3 (solid) ↔ Ca 2+ + CO 3 2-
Solid carbonates turn into soluble bicarbonates. It is this process of chemically dissolving excess carbon dioxide that counteracts the “greenhouse effect” - global warming due to the absorption of thermal radiation from the Earth by carbon dioxide. About a third of the world's production of soda (sodium carbonate Na 2 CO 3) is used in glass production.