ATP, or adenosine triphosphoric acid in full, is an “accumulator” of energy in the cells of the body. No bio chemical reaction does not occur without the participation of ATP. ATP molecules are found in DNA and RNA.
ATP composition
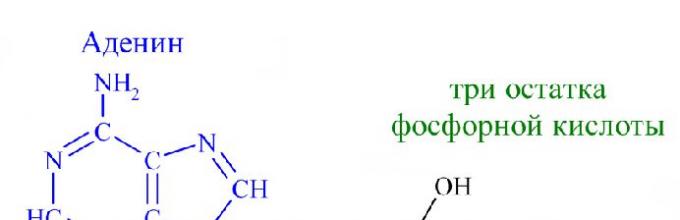
The ATP molecule has three components: three remainders phosphoric acid, adenine and ribose. That is, ATP has the structure of a nucleotide and belongs to nucleic acids. Ribose is a carbohydrate and adenine is a nitrogenous base. The acid residues are united with each other by unstable energetic bonds. Energy appears when acid molecules are broken off. The separation occurs thanks to biocatalysts. After disconnecting, ATP molecule is already converted into ADP (if one molecule has been split off) or into AMP (if two acid molecules have been split off). When one molecule of phosphoric acid is separated, 40 kJ of energy is released.
Role in the body
ATP plays not only an energy role in the body, but also a number of others:
- is the result of the synthesis of nucleic acids.
- regulation of many biochemical processes.
- signaling substance in other cell interactions.
ATP synthesis
ATP production takes place in chloroplasts and mitochondria. The most important process in the synthesis of ATP molecules is dissimilation. Dissimilation is the destruction of the complex into a simpler one.
ATP synthesis occurs not in one stage, but in three stages:
- The first stage is preparatory. Under the action of enzymes in digestion, the breakdown of what we have absorbed occurs. In this case, fats are decomposed into glycerol and fatty acids, proteins into amino acids, and starch into glucose. That is, everything is prepared for further use. Thermal energy released
- The second stage is glycolysis (oxygen-free). Decay occurs again, but here glucose also undergoes decay. Enzymes are also involved. But 40% of the energy remains in ATP, and the rest is consumed as heat.
- The third stage is hydrolysis (oxygen). It already occurs in the mitochondria themselves. Both the oxygen we inhale and enzymes take part here. After complete dissimilation, energy is released for the formation of ATP.
ATP and other cell compounds(vitamins)
Especially important role In the bioenergetics of the cell, the adenyl nucleotide plays a role, to which two phosphoric acid residues are attached. This substance is called adenosine triphosphoric acid(ATP).
IN chemical bonds Between the phosphoric acid residues of the ATP molecule, energy is stored, which is released during the cleavage of organic phosphate: ATP = ADP + P + E, where P is the enzyme, E is the released energy. In this reaction, adenosine diphosphoric acid (ADP) is formed - the remainder of the ATP molecule and organic phosphate.
All cells use ATP energy for the processes of biosynthesis, movement, production of heat, nerve impulses, luminescence (for example, in luminescent bacteria), i.e. for all life processes.
ATP is a universal biological energy accumulator that synthesized in mitochondria (intracellular organelles).
Mitochondria thus plays the role of an “energy station” in the cell. The principle of ATP formation in the chloroplasts of plant cells is generally the same - the use of a proton gradient and the conversion of the energy of the electrochemical gradient into the energy of chemical bonds.
The light energy of the Sun and the energy contained in the food consumed is stored in ATP molecules. The supply of ATP in the cell is small. So, the ATP reserve in the muscle is enough for 20-30 contractions. With enhanced, but short-term work muscles work solely by breaking down the ATP they contain. After finishing work, a person breathes heavily - during this period, carbohydrates and other substances are broken down (energy is accumulated) and the supply of ATP in the cells is restored by protons. Protons pass through this channel under the influence driving force electrochemical gradient. The energy of this process is used by an enzyme contained in the same protein complexes that is capable of attaching a phosphate group to adenosine diphosphate (ADP), which leads to the synthesis of ATP.
Vitamins: Vita - life.
Vitamins - biologically active substances, synthesized in the body or supplied with food, which in small quantities are necessary for normal metabolism and vital functions of the body.
In 1911 The Polish chemist K. Funk isolated a substance from rice bran that cured the paralysis of pigeons that ate only polished rice. Chemical analysis of this substance showed that it contains nitrogen.
Funk called the substance he discovered a vitamin (from the words “vita” - life and “amine” - containing nitrogen.
Biological role of vitamins lies in their regular effect on metabolism. Vitamins have catalytic properties, that is, the ability to stimulate chemical reactions occurring in the body, and also actively participate in the formation and function of enzymes. Vitamins affect absorption nutrients, contribute to normal cell growth and development of the entire organism. Being integral part enzymes, vitamins determine their normal function and activity. Thus, a lack of any vitamin in the body leads to disruption of metabolic processes.
Groups of vitamins:
DAILY REQUIREMENT FOR VITAMINS
C - ascorbic acid: 70 - 100 mg.
B - thiamine: 1.5 - 2.6 mg.
B - riboflavin: 1.8 - 3 mg.
A - retinol: 1.5 mg.
D - calciferol: for children and adults 100 IU,
up to 3 years 400 IU.
E - tocopherol: 15 - 20 mg.
In biology, ATP is the source of energy and the basis of life. ATP - adenosine triphosphate - is involved in metabolic processes and regulates biochemical reactions in the body.
What is this?
Chemistry will help you understand what ATP is. Chemical formula ATP molecules - C10H16N5O13P3. Remembering the full name is easy if you break it down into its component parts. Adenosine triphosphate or adenosine triphosphoric acid is a nucleotide consisting of three parts:
- adenine - purine nitrogenous base;
- ribose - a monosaccharide related to pentoses;
- three phosphoric acid residues.

Rice. 1. The structure of the ATP molecule.
A more detailed explanation of ATP is presented in the table.
ATP was first discovered by Harvard biochemists Subbarao, Lohman, and Fiske in 1929. In 1941, German biochemist Fritz Lipmann discovered that ATP is the source of energy for a living organism.
Energy generation
Phosphate groups are interconnected by high-energy bonds that are easily destroyed. During hydrolysis (reaction with water), the bonds of the phosphate group break apart, releasing large number energy, and ATP is converted into ADP (adenosine diphosphoric acid).
Conventionally, the chemical reaction looks like this:
TOP 4 articleswho are reading along with this
ATP + H2O → ADP + H3PO4 + energy

Rice. 2. ATP hydrolysis.
Part of the released energy (about 40 kJ/mol) is involved in anabolism (assimilation, plastic metabolism), while part is dissipated in the form of heat to maintain body temperature. With further hydrolysis of ADP, another phosphate group is split off, releasing energy and forming AMP (adenosine monophosphate). AMP does not undergo hydrolysis.
ATP synthesis
ATP is located in the cytoplasm, nucleus, chloroplasts, and mitochondria. ATP synthesis in animal cell occurs in mitochondria, and in plants - in mitochondria and chloroplasts.
ATP is formed from ADP and phosphate with the expenditure of energy. This process is called phosphorylation:
ADP + H3PO4 + energy → ATP + H2O

Rice. 3. Formation of ATP from ADP.
IN plant cells Phosphorylation occurs during photosynthesis and is called photophosphorylation. In animals, the process occurs during respiration and is called oxidative phosphorylation.
In animal cells, ATP synthesis occurs during the process of catabolism (dissimilation, energy metabolism) during the breakdown of proteins, fats, carbohydrates.
Functions
From the definition of ATP it is clear that this molecule is capable of providing energy. In addition to energy, adenosine triphosphoric acid performs other functions:
- is a material for the synthesis of nucleic acids;
- is part of enzymes and regulates chemical processes, accelerating or slowing down their occurrence;
- is a mediator - transmits a signal to synapses (places of contact between two cell membranes).
What have we learned?
From a 10th grade biology lesson we learned about the structure and functions of ATP - adenosine triphosphoric acid. ATP consists of adenine, ribose and three phosphoric acid residues. During hydrolysis, phosphate bonds are broken, which releases the energy necessary for the life of organisms.
Test on the topic
Evaluation of the report
Average rating: 4.6. Total ratings received: 621.
ATP is the abbreviation for Adenosine Tri-Phosphoric Acid. You can also find the name Adenosine triphosphate. This is a nucleoid that plays a huge role in energy exchange in the body. Adenosine Tri-Phosphoric acid is a universal source of energy involved in all biochemical processes of the body. This molecule was discovered in 1929 by the scientist Karl Lohmann. And its significance was confirmed by Fritz Lipmann in 1941.
Structure and formula of ATP
If we talk about ATP in more detail, then this is a molecule that provides energy to all processes occurring in the body, including the energy for movement. When the ATP molecule is broken down, the muscle fiber contracts, resulting in the release of energy that allows contraction to occur. Adenosine triphosphate is synthesized from inosine in a living organism.
In order to give the body energy, adenosine triphosphate must go through several stages. First, one of the phosphates is separated using a special coenzyme. Each phosphate provides ten calories. The process produces energy and produces ADP (adenosine diphosphate).
If the body needs more energy to function, then another phosphate is separated. Then AMP (adenosine monophosphate) is formed. The main source for the production of Adenosine Triphosphate is glucose; in the cell it is broken down into pyruvate and cytosol. Adenosine triphosphate energizes long fibers that contain the protein myosin. It is what forms muscle cells.
At moments when the body is resting, the chain goes into reverse side, i.e. Adenosine Tri-Phosphoric acid is formed. Again, glucose is used for these purposes. The created Adenosine Triphosphate molecules will be reused as soon as necessary. When energy is not needed, it is stored in the body and released as soon as it is needed.
The ATP molecule consists of several, or rather, three components:
- Ribose is a five-carbon sugar that forms the basis of DNA.
- Adenine is the combined atoms of nitrogen and carbon.
- Triphosphate.
At the very center of the adenosine triphosphate molecule is a ribose molecule, and its edge is the main one for adenosine. On the other side of ribose is a chain of three phosphates.
ATP systems
 At the same time, you need to understand that ATP reserves will only be sufficient for the first two or three seconds motor activity, after which its level decreases. But at the same time, muscle work can only be carried out with the help of ATP. Thanks to special systems in the body, new ATP molecules are constantly synthesized. The inclusion of new molecules occurs depending on the duration of the load.
At the same time, you need to understand that ATP reserves will only be sufficient for the first two or three seconds motor activity, after which its level decreases. But at the same time, muscle work can only be carried out with the help of ATP. Thanks to special systems in the body, new ATP molecules are constantly synthesized. The inclusion of new molecules occurs depending on the duration of the load.
ATP molecules synthesize three main biochemical systems:
- Phosphagen system (creatine phosphate).
- Glycogen and lactic acid system.
- Aerobic respiration.
Let's consider each of them separately.
Phosphagen system- if the muscles work for a short time, but extremely intensely (about 10 seconds), the phosphagen system will be used. In this case, ADP binds to creatine phosphate. Thanks to this system, a small amount of Adenosine Triphosphate is constantly circulated in muscle cells. Since the muscle cells themselves also contain creatine phosphate, it is used to restore ATP levels after high-intensity short work. But within ten seconds the level of creatine phosphate begins to decrease - this energy is enough for a short race or intense strength training in bodybuilding.
 Glycogen and lactic acid- supplies energy to the body more slowly than the previous one. It synthesizes ATP, which can be enough for one and a half minutes of intense work. In the process, glucose in muscle cells is formed into lactic acid through anaerobic metabolism.
Glycogen and lactic acid- supplies energy to the body more slowly than the previous one. It synthesizes ATP, which can be enough for one and a half minutes of intense work. In the process, glucose in muscle cells is formed into lactic acid through anaerobic metabolism.
Since in an anaerobic state oxygen is not used by the body, then this system provides energy in the same way as in the aerobic system, but time is saved. In anaerobic mode, muscles contract extremely powerfully and quickly. Such a system can allow you to run a four hundred meter sprint or a longer intense workout in the gym. But working in this way for a long time will not allow muscle soreness, which appears due to an excess of lactic acid.
Aerobic respiration- this system turns on if the workout lasts more than two minutes. Then the muscles begin to receive adenosine triphosphate from carbohydrates, fats and proteins. In this case, ATP is synthesized slowly, but the energy lasts for a long time - physical activity can last for several hours. This happens due to the fact that glucose breaks down without obstacles, it does not have any counteractions from outside - as lactic acid interferes with the anaerobic process.
The role of ATP in the body
From the previous description it is clear that the main role of adenosine triphosphate in the body is to provide energy for all the numerous biochemical processes and reactions in the body. Most energy-consuming processes in living beings occur thanks to ATP.
But besides this main function, adenosine triphosphate also performs others:

The role of ATP in the human body and life is well known not only to scientists, but also to many athletes and bodybuilders, since its understanding helps make training more effective and correctly calculate loads. For people who do strength training in the gym, sprinting and other sports, it is very important to understand what exercises need to be performed at one time or another. Thanks to this, you can form the desired body structure, work out the muscle structure, reduce excess weight and achieve other desired results.
Millions of biochemical reactions take place in any cell of our body. They are catalyzed by a variety of enzymes, which often require energy. Where does the cell get it? This question can be answered if we consider the structure of the ATP molecule - one of the main sources of energy.
ATP is a universal energy source
ATP stands for adenosine triphosphate, or adenosine triphosphate. The substance is one of the two most important sources of energy in any cell. The structure of ATP and biological role closely related. Most biochemical reactions can occur only with the participation of molecules of a substance, this is especially true. However, ATP is rarely directly involved in the reaction: for any process to occur, the energy contained precisely in adenosine triphosphate is needed.
The structure of the molecules of the substance is such that the bonds formed between phosphate groups carry a huge amount of energy. Therefore, such bonds are also called macroergic, or macroenergetic (macro=many, large amount). The term was first introduced by the scientist F. Lipman, and he also proposed using the symbol ̴ to designate them.
It is very important for the cell to maintain a constant level of adenosine triphosphate. This is especially true for muscle cells and nerve fibers, because they are the most energy-dependent and require a high content of adenosine triphosphate to perform their functions.
The structure of the ATP molecule
Adenosine triphosphate consists of three elements: ribose, adenine and residues

Ribose- a carbohydrate that belongs to the pentose group. This means that ribose contains 5 carbon atoms, which are enclosed in a cycle. Ribose connects to adenine through a β-N-glycosidic bond on the 1st carbon atom. Phosphoric acid residues on the 5th carbon atom are also added to pentose.
Adenine is a nitrogenous base. Depending on which nitrogenous base is attached to ribose, GTP (guanosine triphosphate), TTP (thymidine triphosphate), CTP (cytidine triphosphate) and UTP (uridine triphosphate) are also distinguished. All these substances are similar in structure to adenosine triphosphate and perform approximately the same functions, but they are much less common in the cell.
Phosphoric acid residues. A maximum of three phosphoric acid residues can be attached to ribose. If there are two or only one, then the substance is called ADP (diphosphate) or AMP (monophosphate). It is between the phosphorus residues that macroenergetic bonds are concluded, after the rupture of which 40 to 60 kJ of energy is released. If two bonds are broken, 80, less often - 120 kJ of energy is released. When the bond between ribose and the phosphorus residue is broken, only 13.8 kJ is released, so there are only two high-energy bonds in the triphosphate molecule (P ̴ P ̴ P), and in the ADP molecule there is one (P ̴ P).
These are the structural features of ATP. Due to the fact that a macroenergetic bond is formed between phosphoric acid residues, the structure and functions of ATP are interconnected.
The structure of ATP and the biological role of the molecule. Additional functions of adenosine triphosphate
In addition to energy, ATP can perform many other functions in the cell. Along with other nucleotide triphosphates, triphosphate is involved in the construction nucleic acid. In this case, ATP, GTP, TTP, CTP and UTP are suppliers of nitrogenous bases. This property is used in processes and transcription.
ATP is also necessary for the functioning of ion channels. For example, the Na-K channel pumps 3 sodium molecules out of the cell and pumps 2 potassium molecules into the cell. This ion current is needed to maintain a positive charge on the outer surface of the membrane, and only with the help of adenosine triphosphate can the channel function. The same applies to proton and calcium channels.
ATP is the precursor of the second messenger cAMP (cyclic adenosine monophosphate) - cAMP not only transmits the signal received by cell membrane receptors, but is also an allosteric effector. Allosteric effectors are substances that speed up or slow down enzymatic reactions. Thus, cyclic adenosine triphosphate inhibits the synthesis of an enzyme that catalyzes the breakdown of lactose in bacterial cells.
The adenosine triphosphate molecule itself may also be an allosteric effector. Moreover, in such processes, ADP acts as an antagonist to ATP: if triphosphate accelerates the reaction, then diphosphate inhibits it, and vice versa. These are the functions and structure of ATP.

How is ATP formed in a cell?
The functions and structure of ATP are such that the molecules of the substance are quickly used and destroyed. Therefore, triphosphate synthesis is an important process in the formation of energy in the cell.
There are three most important ways of adenosine triphosphate synthesis:
1. Substrate phosphorylation.
2. Oxidative phosphorylation.
3. Photophosphorylation.
Substrate phosphorylation is based on multiple reactions occurring in the cell cytoplasm. These reactions are called glycolysis - anaerobic stage. As a result of 1 cycle of glycolysis, from 1 molecule of glucose two molecules are synthesized, which are then used to produce energy, and two ATP are also synthesized.
- C 6 H 12 O 6 + 2ADP + 2Pn --> 2C 3 H 4 O 3 + 2ATP + 4H.
Cell respiration
Oxidative phosphorylation is the formation of adenosine triphosphate by transferring electrons along the membrane electron transport chain. As a result of this transfer, a proton gradient is formed on one side of the membrane and, with the help of the protein integral set of ATP synthase, molecules are built. The process takes place on the mitochondrial membrane.

The sequence of stages of glycolysis and oxidative phosphorylation in mitochondria constitutes a common process called respiration. After a complete cycle, 36 ATP molecules are formed from 1 glucose molecule in the cell.
Photophosphorylation
The process of photophosphorylation is the same oxidative phosphorylation with only one difference: photophosphorylation reactions occur in the chloroplasts of the cell under the influence of light. ATP is produced during the light stage of photosynthesis, the main energy production process in green plants, algae and some bacteria.
During photosynthesis, electrons pass through the same electron transport chain, resulting in the formation of a proton gradient. The concentration of protons on one side of the membrane is the source of ATP synthesis. The assembly of molecules is carried out by the enzyme ATP synthase.

The average cell contains 0.04% adenosine triphosphate by weight. However, the most great value observed in muscle cells: 0.2-0.5%.
There are about 1 billion ATP molecules in a cell.
Each molecule lives no more than 1 minute.
One molecule of adenosine triphosphate is renewed 2000-3000 times a day.
In total, the human body synthesizes 40 kg of adenosine triphosphate per day, and at any given time the ATP reserve is 250 g.

Conclusion
The structure of ATP and the biological role of its molecules are closely related. The substance plays a key role in life processes, because the high-energy bonds between phosphate residues contain a huge amount of energy. Adenosine triphosphate performs many functions in the cell, and therefore it is important to maintain a constant concentration of the substance. Decay and synthesis occur at high speed, since the energy of bonds is constantly used in biochemical reactions. This is an essential substance for any cell in the body. That's probably all that can be said about the structure of ATP.