- managed. By the control mechanism: electro-, chemo- and mechanically controlled;
- uncontrollable. They do not have a gate mechanism and are always open, ions go constantly, but slowly.
Resting potential Is the difference in electrical potentials between the external and internal environment cells.
Resting potential formation mechanism. The immediate cause of the resting potential is the unequal concentration of anions and cations inside and outside the cell. First, such an arrangement of ions is based on the difference in permeability. Secondly, much more potassium ions leave the cell than sodium.
Action potential - this is the excitation of the cell, a rapid fluctuation in the membrane potential due to the diffusion of ions into and out of the cell.
When the stimulus acts on the cells of the excitable tissue, at first, sodium channels are very quickly activated and inactivated, then, with some delay, potassium channels are activated and inactivated.
As a consequence, ions rapidly diffuse into or out of the cell according to an electrochemical gradient. This is excitement. According to the change in the magnitude and sign of the cell charge, three phases are distinguished:
- 1st phase - depolarization. Reducing the cell charge to zero. Sodium moves to the cell according to the concentration and electrical gradient. Condition of movement: the gate of the sodium channel is open;
- 2nd phase - inversion. Reversal of charge sign. Inversion involves two parts: upward and downward.
Ascending part. Sodium continues to move into the cell according to the concentration gradient, but contrary to the electrical gradient (it interferes).
Descending part. Potassium begins to leave the cell according to the concentration and electrical gradient. The gates of the potassium channel are open;
- 3rd phase - repolarization. Potassium continues to leave the cell according to the concentration, but contrary to the electrical gradient.
Criteria for excitability
With the development of the action potential, a change in tissue excitability occurs. This change occurs in phases. The state of the initial membrane polarization characteristically reflects the resting membrane potential, which corresponds to the initial state of excitability and, consequently, the initial state of the excitable cell. This is a normal level of excitability. The pre-soldering period is the period of the very beginning of the action potential. Tissue excitability is slightly increased. This phase of excitability is primary exaltation (primary supernormal excitability). During the development of the prejunction, the membrane potential approaches the critical level of depolarization, and to reach this level, the strength of the stimulus may be less than the threshold.
During the development of the spike (peak potential), an avalanche-like flow of sodium ions into the cell occurs, as a result of which the membrane recharges, and it loses its ability to respond with excitation to stimuli of a suprathreshold force. This phase of excitability is called absolute refractoriness, i.e. absolute non-excitability, which lasts until the end of the membrane recharge. The absolute refractoriness of the membrane occurs due to the fact that sodium channels are completely opened and then inactivated.
After the end of the recharge phase, its excitability is gradually restored to its original level - this is the phase of relative refractoriness, i.e. relative non-excitability. It continues until the membrane charge is restored to a value corresponding to the critical level of depolarization. Since during this period the membrane potential of rest has not yet been restored, the excitability of the tissue is reduced, and new excitation can arise only under the action of a suprathreshold stimulus. A decrease in excitability in the phase of relative refractoriness is associated with partial inactivation of sodium channels and activation of potassium channels.
The next period corresponds to an increased level of excitability: the phase of secondary exaltation or secondary supernormal excitability. Since the membrane potential in this phase is closer to the critical level of depolarization, in comparison with the resting state of the initial polarization, the stimulation threshold is reduced, i.e. the excitability of the cell is increased. In this phase, new excitement can arise under the action of stimuli of the subthreshold force. Sodium channels in this phase are not completely inactivated. The membrane potential increases - a state of membrane hyperpolarization occurs. Moving away from the critical level of depolarization, the threshold of irritation rises slightly, and new excitation can arise only under the action of stimuli of a suprathreshold magnitude.
The mechanism of the resting membrane potential
Each cell at rest is characterized by the presence of a transmembrane potential difference (resting potential). Typically, the difference in charge between the inner and outer surfaces of membranes is from -80 to -100 mV and can be measured using the outer and intracellular microelectrodes (Fig. 1).
The potential difference between the outer and inner sides of the cell membrane in its resting state is called membrane potential (resting potential).
The creation of resting potential is provided by two main processes - the uneven distribution of inorganic ions between the intra- and extracellular spaces and the unequal permeability of the cell membrane for them. Analysis of the chemical composition of the extracellular and intracellular fluid indicates an extremely uneven distribution of ions (Table 1).
At rest, inside the cell there are many organic acid anions and K + ions, the concentration of which is 30 times higher than outside; Na + ions, on the contrary, are 10 times more outside the cell than inside; CI- is also larger on the outside.
At rest, the membrane of nerve cells is most permeable for K +, less for CI- and very little permeable for Na + / The permeability of the nerve fiber membrane for Na + B at rest is 100 times less than for K +. For many anions of organic acids, the membrane at rest is completely impenetrable.
Figure: 1. Measurement of the resting potential of muscle fibers (A) using an intracellular microelectrode: M - microelectrode; And - an indifferent electrode. The beam on the oscilloscope screen (B) shows that before the membrane was pierced by the microelectrode, the potential difference between M and I was zero. At the moment of puncture (shown by an arrow), a potential difference was detected, indicating that the inner side of the membrane is charged negatively with respect to its outer surface (according to B.I.Khodorov)
Table. Intra- and extracellular concentrations of muscle cell ions in a warm-blooded animal, mmol / l (according to J. Dudel)
|
Intracellular concentration |
Extracellular concentration |
|
|
A- (anions of organic compounds) |
Due to the concentration gradient, K + enters the outer surface of the cell, carrying out its positive charge. High molecular weight anions cannot follow K + because of their impermeability to the membrane. The Na + ion also cannot replace the left potassium ions, because the membrane permeability for it is much lower. СI- along the concentration gradient can mix only inside the cell, thereby increasing the negative charge of the inner surface of the membrane. Due to this movement of ions, polarization of the membrane occurs when its outer surface is charged positively, and its inner surface is negatively charged.
The electric field that is created on the membrane actively interferes with the distribution of ions between the inner and outer contents of the cell. As the positive charge on the outer surface of the cell increases, the K + ion, as a positively charged one, becomes more and more difficult to move from the inside to the outside. It moves uphill, as it were. The greater the value of the positive charge on the outer surface, the smaller the amount of K + ions can go to the cell surface. At a certain value of the potential on the membrane, the number of K + ions crossing the membrane in either direction turns out to be equal, i.e. the potassium concentration gradient is balanced by the potential on the membrane. The potential at which the diffusion flux of ions becomes equal to the flux of like ions going in the opposite direction is called the equilibrium potential for a given ion. For K + ions, the equilibrium potential is -90 mV. In myelinated nerve fibers, the value of the equilibrium potential for CI- ions is close to the value of the resting membrane potential (-70 mV). Therefore, despite the fact that the concentration of СI- ions outside the fiber is greater than inside it, their one-way current is not observed in accordance with the concentration gradient. In this case, the concentration difference is balanced by the potential on the membrane.
The Na + ion along the concentration gradient would have to enter the cell (its equilibrium potential is +60 mV), and the presence of a negative charge inside the cell should not impede this flow. In this case, the incoming Na + would neutralize the negative charges inside the cell. However, this does not actually happen, since the membrane at rest is poorly permeable to Na +.
The most important mechanism supporting a low intracellular concentration of Na + ions and a high concentration of K + ions is the sodium-potassium pump (active transport). It is known that there is a system of carriers in the cell membrane, each of which binds to the stirrup by the Na + ions inside the cell and removes them outside. From the outside, the carrier binds to two K + ions outside the cell, which are carried into the cytoplasm. The power supply for the operation of the carrier systems is provided by ATP. The operation of the pump on such a system leads to the following results:
- a high concentration of K + ions is maintained inside the cell, which ensures the constancy of the resting potential. Due to the fact that in one cycle of ion exchange one more positive ion is removed from the cell than is introduced, active transport plays a role in creating resting potential. In this case, they speak of an electrogenic pump, since it itself creates a small, but d.C. positive charges from the cell, and therefore makes a direct contribution to the formation of a negative potential inside it. However, the contribution of the electrogenic pump to the total value of the resting potential is usually small and amounts to several millivolts;
- a low concentration of Na + ions inside the cell is maintained, which, on the one hand, ensures the operation of the mechanism for generating an action potential, on the other hand, ensures the maintenance of normal osmolarity and cell volume;
- maintaining a stable concentration gradient of Na +, the sodium-potassium pump promotes the conjugated K +, Na + -transport of amino acids and sugars across the cell membrane.
Thus, the emergence of a transmembrane potential difference (resting potential) is due to the high conductivity of the cell membrane at rest for K +, CI- ions, ionic asymmetry of the concentrations of K + ions and CI- ions, the work of active transport systems (Na + / K + -ATPase), which create and maintain ionic asymmetry.
Nerve fiber action potential, nerve impulse
Action potential - this is a short-term fluctuation in the potential difference of the membrane of an excitable cell, accompanied by a change in its charge sign.
Action potential is the main specific sign of arousal. Its registration indicates that the cell or its structures responded to the stimulation. However, as already noted, PD in some cells can occur spontaneously (spontaneously). Such cells are contained in the pacemakers of the heart, the walls of blood vessels, nervous system... PD is used as a carrier of information, transmitting it in the form of electrical signals (electrical signaling) along afferent and efferent nerve fibers, the conduction system of the heart, as well as to initiate contraction of muscle cells.
Let us consider the causes and mechanism of AP generation in afferent nerve fibers that form primary sensory receptors. The immediate cause of the onset (generation) of AP in them is the receptor potential.
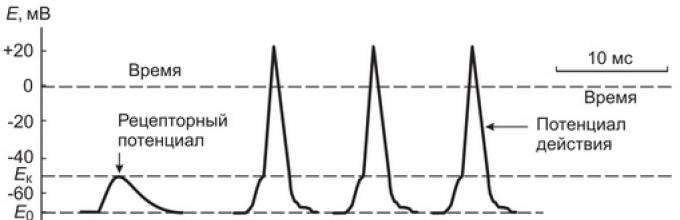
If we measure the potential difference on the membrane of the Ranvier interception closest to the nerve ending, then in the intervals between the impacts on the capsule of the Pacini corpuscle it remains unchanged (70 mV), and during the impact it depolarizes almost simultaneously with the depolarization of the receptor membrane of the nerve ending.
With an increase in the pressure force on the Pacini body, causing an increase in the receptor potential up to 10 mV, in the nearest interception of Ranvier, a rapid oscillation of the membrane potential is usually recorded, accompanied by membrane recharge - the action potential (AP), or a nerve impulse (Fig. 2). If the force of pressure on the body increases even more, the amplitude of the receptor potential increases and a number of action potentials are generated in the nerve ending with a certain frequency.

Figure: 2. Schematic representation of the mechanism of converting the receptor potential into an action potential (nerve impulse) and propagation of the impulse along the nerve fiber
The essence of the mechanism of AP generation is that the receptor potential causes the emergence of local circular currents between the depolarized receptor membrane of the unmyelinated part of the nerve ending and the membrane of the first interception of Ranvier. These currents, which are carried by the ions Na +, K +, CI- and other mineral ions, "flow" not only along, but also across the membrane of the nerve fiber in the area of \u200b\u200binterception of Ranvier. In the membrane of Ranvier's interceptions, in contrast to the receptor membrane of the nerve ending itself, there is a high density of ionic voltage-dependent sodium and potassium channels.
When the depolarization value of about 10 mV is reached on the membrane of the Ranvier interception, fast voltage-dependent sodium channels are opened and through them the flow of Na + ions rushes into the axoplasm along the electrochemical gradient. It causes rapid depolarization and recharge of the Ranvier interception membrane. However, simultaneously with the opening of fast voltage-gated sodium channels, slow voltage-gated potassium channels open in the Ranvier interception membrane and K + ions begin to leave the axoilasm. Thus, the Na + ions entering the axoplasm at a high rate quickly depolarize and recharge the membrane for a short time (0.3-0.5 ms), while the outgoing K + ions restore the original distribution of charges on the membrane (repolarize the membrane). As a result, during the mechanical action on the Pacini body by a force equal to or exceeding the threshold, a short-term fluctuation of potential is observed on the membrane of the nearest interception of Ranvier in the form of rapid depolarization and repolarization of the membrane, i.e. PD (nerve impulse) is generated.
Since the immediate cause of AP generation is the receptor potential, in this case it is also called the generator potential. The number of nerve impulses of the same amplitude and duration generated per unit time is proportional to the amplitude of the receptor potential, and therefore to the force of pressure on the receptor. The process of converting information about the strength of the impact, embedded in the amplitude of the receptor potential, into the number of discrete nerve impulses is called discrete coding of information.
The ionic mechanisms and temporal dynamics of the processes of AP generation have been studied in more detail under experimental conditions under artificial action on the nerve fiber with electric current of various strength and duration.
The nature of the action potential of the nerve fiber (nerve impulse)
The membrane of the nerve fiber at the point of localization of the irritating electrode responds to the effect of a very weak current, which has not yet reached the threshold value. This answer is called local, and the fluctuation of the potential difference across the membrane is called local potential.
A local response on the membrane of an excitable cell can precede the emergence of an action potential or arise as an independent process. It is a short-term fluctuation (depolarization and repolarization) of the resting potential, not accompanied by membrane recharge. Depolarization of the membrane during the development of the local potential is due to the advanced entry into the axoplasm of Na + ions, and repolarization is due to the delayed exit from the axoplasm of K + ions.
If you act on the membrane with an electric current of increasing force, then at this value, called the threshold, the depolarization of the membrane can reach a critical level - E k, at which fast voltage-dependent sodium channels open. As a result, through them there is an avalanche-like increasing flow of Na + ions into the cell. The evoked process of depolarization acquires a self-accelerating character, and the local potential develops into an action potential.
It has already been mentioned that characteristic feature PD is a short-term inversion (change) of the sign of the charge on the membrane. Outside, it becomes negatively charged for a short time (0.3-2 ms), and inside - positively. The magnitude of the inversion can be up to 30 mV, and the magnitude of the entire action potential - 60-130 mV (Fig. 3).
Table. Comparative characteristics local potential and action potential
|
Characteristic |
Local potential |
Action potential |
|
Conductivity |
It spreads locally, by 1-2 mm with attenuation (decrement) |
Spreads without attenuation over long distances along the entire length of the nerve fiber |
|
The law of "force" |
Submits |
Does not obey |
|
All or Nothing Law |
Does not obey |
Submits |
|
Summation phenomenon |
It is cumulative, increases with repeated frequent subthreshold irritations |
Not cumulative |
|
Amplitude |
||
|
The ability to excitability |
Increases |
Decreases up to complete non-excitability (refractoriness) |
|
Stimulus magnitude |
Subthreshold |
Threshold and suprathreshold |
The action potential, depending on the nature of the change in charges on the inner surface of the membrane, is divided into phases of depolarization, repolarization and hyperpolarization of the membrane. Depolarization call the entire ascending part of the AP, on which the areas corresponding to the local potential (from the level E 0 before E to), rapid depolarization (from the level E to to the level of 0 mV), inversions the sign of the charge (from 0 mV to the peak value or the beginning of repolarization). Repolarization is called the descending part of the AP, which reflects the process of restoration of the initial membrane polarization. At first, repolarization is carried out quickly, but approaching the level E 0, the speed can slow down and this section is called trace negativity (or trace negative potential). In some cells, following repolarization, hyperpolarization (an increase in membrane polarization) develops. They call her trace positive potential.
The initial high-amplitude fast-flowing part of the PD is also called peak, or spike. It includes phases of depolarization and rapid repolarization.
In the mechanism of PD development, the most important role is played by voltage-dependent ion channels and a non-simultaneous increase in the permeability of the cell membrane for Na + and K + ions. So, when an electric current acts on a cell, it causes membrane depolarization, and when the membrane charge decreases to a critical level (E to), voltage-dependent sodium channels open. As already mentioned, these channels are formed by protein molecules embedded in the membrane, within which there is a pore and two gate mechanisms. One of the gate mechanisms - the activation one, provides (with the participation of segment 4) the opening (activation) of the channel during membrane depolarization, and the second (with the participation of the intracellular loop between the 3rd and 4th domains) - its inactivation, which develops during membrane recharging (Fig. 4). Since both of these mechanisms rapidly change the position of the channel gates, voltage-gated sodium channels are fast ion channels. This circumstance is of decisive importance for the generation of AP in excitable tissues and for its conduction through the membranes of nerve and muscle fibers.

Figure: 3. Action potential, its phases and ionic currents (a, o). Description in text

Figure: 4. Position of the gate and the state of activity of voltage-gated sodium and potassium channels at different levels of membrane polarization
In order for the voltage-gated sodium channel to pass Na + ions into the cell, it is only necessary to open the activation gate, since the inactivation gates are open at rest. This happens when membrane depolarization reaches the level E to (Fig. 3, 4).
Opening the activation gates of sodium channels leads to an avalanche-like entry of sodium into the cell, driven by the action of the forces of its electrochemical gradient. Since Na + ions carry a positive charge, they neutralize the excess of negative charges on the inner surface of the membrane, reduce the potential difference across the membrane and depolarize it. Soon, Na + ions impart an excess of positive charges to the inner surface of the membrane, which is accompanied by an inversion (change) of the charge sign from negative to positive.
However, sodium channels remain open only for about 0.5 ms, and after this period of time from the moment of the beginning
AP closes the inactivation gate, sodium channels become inactivated and impermeable to Na + ions, the entry of which into the cell is sharply limited.
From the moment of membrane depolarization to the level E to activation of potassium channels and opening of their gates for K + ions are also observed. K + ions, under the action of the forces of the concentration gradient, leave the cell, carrying out positive charges from it. However, the gate mechanism of potassium channels is slowly functioning and the rate of release of positive charges with K + ions from the cell to the outside is delayed in relation to the entrance of Na + ions. The flow of K + ions, removing the excess of positive charges from the cell, causes the restoration of the initial distribution of charges on the membrane or its repolarization, and a negative charge is restored on the inner side after a moment from the moment of recharge.
The appearance of AP on excitable membranes and the subsequent restoration of the initial resting potential on the membrane are possible because the dynamics of the entry into and exit from the cell of positive charges of Na + and K + ions are different. The entry of the Na + ion is ahead of the exit of the K + ion in time. If these processes were in equilibrium, then the potential difference across the membrane would not change. The development of the ability to excite and generate AP by excitable muscle and nerve cells was due to the formation in their membrane of two types of different-speed ion channels - fast sodium and slow potassium.
The generation of a single AP requires the entry into the cell of a relatively small number of Na + ions, which does not disrupt its distribution outside and inside the cell. When a large number of APs are generated, the distribution of ions on both sides of the cell membrane could be disturbed. However, under normal conditions this is prevented by the operation of the Na +, K + pump.
Under natural conditions, in CNS neurons, the action potential primarily arises in the area of \u200b\u200bthe axonal hillock, in afferent neurons - in the Ranvier interception of the nerve ending closest to the sensory receptor, i.e. in those areas of the membrane where there are fast selective voltage-dependent sodium channels and slow potassium channels. In other types of cells (for example, pacemaker, smooth myocytes), not only sodium and potassium, but also calcium channels play a role in the development of AP.
The mechanisms of perception and conversion of signals into AP in secondary sensory sensory receptors differ from the mechanisms analyzed for primary sensory receptors. In these receptors, the perception of signals is carried out by specialized neurosensory (photoreceptor, olfactory) or sensorepithelial (taste, auditory, vestibular) cells. Each of these sensitive cells has its own, special mechanism for perceiving signals. However, in all cells, the energy of the perceived signal (stimulus) is converted into fluctuations in the potential difference of the plasma membrane, i.e. into the receptor potential.
Thus, the key moment in the mechanisms of transformation of perceived signals into receptor potential by sensory cells is the change in the permeability of ion channels in response to exposure. The opening of Na +, Ca 2+, K + -ion channels during signal perception and transformation is achieved in these cells with the participation of G-proteins, second intracellular mediators, binding to ligands, and phosphorylation of ion channels. As a rule, the receptor potential that has arisen in sensory cells causes the release of a neurotransmitter from them into the synaptic cleft, which ensures the transmission of a signal to the postsynaptic membrane of the afferent nerve ending and the generation of a nerve impulse on its membrane. These processes are detailed in the chapter on sensory systems.
The action potential can be characterized by the amplitude and duration, which for the same nerve fiber remain the same when AP propagates along the fiber. Therefore, the action potential is called discrete potential.
There is a definite connection between the nature of the effect on sensory receptors and the number of APs arising in the afferent nerve fiber in response to the effect. It lies in the fact that for large but strength or duration of exposure in the nerve fiber is formed more nerve impulses, i.e. with an increase in the impact, impulses of a higher frequency will be sent to the nervous system from the receptor. The processes of converting information about the nature of the impact into the frequency and other parameters of nerve impulses transmitted to the central nervous system are called discrete coding of information.
For an explanation the origin of the resting potential were suggested various theories... At the origins of the modern understanding of this problem is the work of V. Yu. Chagovets, who in 1896, as a medical student, expressed the idea of \u200b\u200bthe ionic nature of bioelectric processes and made an attempt to apply Arrhenius's theory of electrolytic dissociation to explain the origin of these potentials. Later, in 1902, Yuri Bernstein developed the membrane-ion theory, which was modified and experimentally substantiated by A. Hodgkin and A. Huxley (1952) and is now widely recognized. According to this theory, bioelectric potentials are due to unequal concentration of K ионов, Na ֹ, Cl "ions inside and outside the cell and different permeability of the surface membrane for them.
The protoplasm of nerve and muscle cells contains 30-50 times more potassium ions, 8-10 times less sodium ions and 50 times less chlorine ions than the extracellular fluid.
An obstacle to the rapid equalization of this concentration difference is the thinnest (about 100 Å) plasma membrane that covers living cells.
|
The structure of this membrane is based on data obtained by electron microscopy, optical microscopy, X-ray diffraction, and chemical analysis. It is assumed that the membrane consists of a double layer of phospholipid molecules, covered from the inside with a layer of protein molecules, and from the outside with a layer of complex carbohydrate molecules - mucopolysaccharides. The three-layer membrane structure is shown schematically in fig. 116. Figure: 116. Diagram of the molecular structure of the membrane. Shown is a bimolecular lipid layer Z (circles indicate the polar groups of phospholipids) and two non-lipid monolayers: outer - mucopolysaccharide - X, inner - protein - Y (according to Robertson). |
The cell membrane contains the finest tubules - "pores" with a diameter of several angstroms. Through these tubules, molecules of water and other substances, as well as ions with a diameter corresponding to the pore size, enter the cell and leave it.
Personal ions are fixed on the structural elements of the membrane, which gives the walls of its pores one or another charge and thereby complicates or facilitates the passage of ions through them. Thus, it is assumed that the presence of dissociated phosphate and carboxyl groups in the membrane is the reason that the membrane of nerve fibers is much less permeable to anions than to cations.
The membrane permeability for different cations is also not the same, and it naturally changes under different functional states of the tissue. At rest, the membrane of nerve fibers is approximately 20-100 times more permeable to K ֹ ions than to Na ions, and upon excitation, sodium permeability begins to significantly exceed the potassium permeability of the membrane.
In order to understand the mechanism of the appearance of the resting membrane potential from the point of view of the Bernstein - Hodgkin theory, let us consider a model experiment. The first half of the vessel ( fig. 117), separated by an artificial semi-permeable membrane, the pores of which freely pass positively charged K ֹ ions and do not pass negatively charged SO "4 ions, are filled with a concentrated K2SO4 solution, and the left half is also filled with a K2SO4 solution, but of a lower concentration.
 |
Due to the existence of a concentration gradient, K ֹ ions will begin to diffuse through the membrane predominantly from the right half of the vessel (where their concentration is C1) to the left (with a C2 concentration). Correspondingly, negatively charged SO "4 anions, for which the membrane is impermeable, will concentrate in the right half of the vessel at the membrane surface. With their negative charge, they will electrostatically hold K ions on the left side of the membrane surface. As a result, the membrane becomes polarized: a potential difference arises between its two surfaces. Figure: 117. The emergence of a potential difference on an artificial membrane separating solutions of K2SO4 of different concentrations (C1 and C2). The membrane is selectively permeable to K ֹ cations (small circles) and does not allow the SO "4 anions (large circles) to pass through. 1 and 2 - electrodes immersed in the solution; 3 - electrical measuring device. |
If now the electrodes are lowered into the right and left halves of the vessel, then the electric measuring device will detect the presence of a potential difference, while the solution with a lower concentration of K2SO4 ions, into which the diffusion of positively charged K ֹ ions occurs predominantly, acquires a positive charge with respect to the solution with a higher concentration of K2SO4.
The potential difference (E) in the considered case can be calculated using the Nernst formula:

There are many reasons to believe that similar relationships take place in the living nerve fiber, since the concentration of K ֹ ions in the protoplasm is more than 30 roses higher than the concentration of these ions in the external solution, and organic (protein, etc.) anions of protoplasm through the membrane practically do not penetrate.
In a state of physiological rest, the diffusion of positively charged K ions from protoplasm into the external liquid gives the outer surface of the membrane a positive charge, and the inner one - a negative one.
An important argument in favor of the correctness of this idea was the fact that the potential difference calculated by the Nernst formula between the outer and inner sides of the muscle fiber membrane (about 90 mV) turned out to be close to that measured in experiments using an intracellular microelectrode.
It was also found that an increase in the concentration of K ֹ ions in the external environment of the cell, and, consequently, a decrease in the difference in the concentration of these ions on both sides of the membrane lead to a drop in the resting potential, and in a certain concentration range these shifts quantitatively well coincide with those calculated by the Nernst formula.
However, the most important, direct, proofs of the correctness of these concepts were obtained by A. Hodzhkin and co-workers (1962) in experiments with the replacement of protoplasm in the giant nerve fibers of the squid mollusk with saline solutions. The protoplasm was carefully squeezed out of a fiber having a diameter of about 1 ml, and the collapsed shell was filled with an artificial saline solution.
In the case when the concentration of potassium ions in this solution was close to the intracellular one, a potential difference was established between the inner and outer sides of the membrane, approximately equal to the resting potential of a normal fiber (50-80 mV). A decrease in the concentration of K ֹ ions in the internal solution led to a natural decrease or even distortion of the resting potential.
Such experiments showed that the concentration gradient of K ions is indeed the main factor determining the value of the resting potential of the nerve fiber.
Along with K ions, Na ions, which diffuse into the protoplasm from the extracellular fluid, where their concentration is high, also participate in the appearance of the rest potential. This diffusion is greatly hampered by the low sodium permeability of the membrane at rest. Nevertheless, diffusing through the membrane into the protoplasm, Na ions transfer their positive charges here, which somewhat reduces the value of the resting potential created by diffusion of K ions from the cell. This explains the fact that the resting potential of most nerve cells and fibers is not 90 mV, as would be expected if this potential was created only by K ions, but 60-70 mV.
Thus, the value of the resting potential of nerve fibers and cells is determined by the ratio of the number of positively charged K ֺ ions, diffusing per unit time from the cell outward, and positively charged Na ions, diffusing through the membrane in the opposite direction. The higher this ratio, the greater the value of the resting potential, and vice versa.
text_fields
text_fields
arrow_upward
Resting Membrane Potential (MPP) or resting potential (PP) is the potential difference of a resting cell between the inner and outer sides of the membrane. The inner side of the cell membrane is negatively charged with respect to the outer one. Taking the potential of the external solution as zero, the MPP is written with a minus sign. The quantity WFPdepends on the type of fabric and varies from -9 to -100 mV. Therefore, at rest, the cell membrane polarized.A decrease in the MPP value is called depolarization,increase - hyperpolarization,restoring the original value WFP- repolarizationmembranes.
Basic provisions of the membrane theory of origin WFPboil down to the following. At rest, the cell membrane is well permeable to K + ions (in some cells and to SG), less permeable to Na + and practically impermeable to intracellular proteins and other organic ions. K + ions diffuse from the cell along a concentration gradient, while non-penetrating anions remain in the cytoplasm, providing the appearance of a potential difference across the membrane.
The arising potential difference prevents the exit of K + from the cell, and at a certain value of it, equilibrium occurs between the exit of K + along the concentration gradient and the entry of these cations along the arisen electrical gradient. The membrane potential at which this equilibrium is achieved is called equilibrium potentialscarlet.Its value can be calculated from the Nernst equation:
where E to- equilibrium potential for TO + ; R - gas constant; T- absolute temperature; F - faraday number; p- valence K + (+1), [K n +] - [K + ext] -external and internal concentration K + -
If we go from natural logarithms to decimal ones and substitute the numerical values \u200b\u200bof constants into the equation, then the equation will take the form: 
In spinal neurons (Table 1.1) E k \u003d -90 mV. The MPP value measured with microelectrodes is noticeably lower - 70 mV.
Table 1.1... Concentration of some ions inside and outside of mammalian spinal motoneurons
| And he |
Concentration |
(mmol / l H 2 O) |
Equilibrium potential (mv) |
|
inside the cell |
outside the cage |
||
| Na + | 15,0 | 150,0 | |
| K + | 150,0 | 5,5 | |
| Сl - | 125,0 | ||
|
Resting membrane potential \u003d -70 mV |
|||
If the membrane potential of a cell is of a potassium nature, then, in accordance with the Nernst equation, its value should linearly decrease with a decrease in the concentration gradient of these ions, for example, with an increase in the concentration of K + in the extracellular fluid. However, the linear dependence of the BMP (Resting Membrane Potential) on the K + concentration gradient exists only when the K + concentration in the extracellular fluid is above 20 mM. At lower concentrations of K + outside the cell, the curve of the dependence of E m on the logarithm of the ratio of the concentration of potassium outside and inside the cell differs from the theoretical one. It is possible to explain the established deviations of the experimental dependence of the magnitude of the MPP and the concentration gradient K + theoretically calculated using the Nernst equation, assuming that the MPP of excitable cells is determined not only by potassium, but also by sodium and chlorine equilibrium potentials. Reasoning similarly to the previous one, you can write:
The values \u200b\u200bof the sodium and chloride equilibrium potentials for spinal neurons (Table 1.1) are +60 and -70 mV, respectively. The E Cl value is equal to the MPP value. This indicates a passive distribution of chlorine ions across the membrane in accordance with chemical and electrical gradients. For sodium ions, the chemical and electrical gradients are directed into the cell.
The contribution of each of the equilibrium potentials to the MPP value is determined by the ratio between the permeability of the cell membrane for each of these ions. The calculation of the value of the membrane potential is carried out using the Goldmann equation:

E m- membrane potential; R- gas constant; T- absolute temperature; F- Faraday number; R K, P Naand R Cl -membrane permeability constants for K + Na + and Cl, respectively; [TO + n ], [ K + ext, [ Na + n [ Na + ext], [Сl - n] and [Сl - int] - concentrations of K +, Na + and Cl outside (n) and inside (outside) cells.
Substituting into this equation the concentration of ions and the value of the MPP obtained in experimental studies, it can be shown that for the giant axon of the squid there should be the following ratio of the permeability constants P to: P Na: P C1 \u003d I: 0.04: 0.45. Obviously, since the membrane is permeable to sodium ions (P N a =/ 0) and the equilibrium potential for these ions has a "plus" sign, then the entry of the latter into the cell along chemical and electrical gradients will reduce the electronegativity of the cytoplasm, i.e. increase the MPP (Resting Membrane Potential).
With an increase in the concentration of potassium ions in the external solution above 15 mM, the MPP increases and the ratio of the permeability constants changes towards a more significant excess of »P to over P Na and P C1. P to: P Na: P C1 \u003d 1: 0.025: 0.4. Under such conditions, the MPP is determined almost exclusively by the gradient of potassium ions; therefore, the experimental and theoretical dependences of the MPP value on the logarithm of the ratio of potassium concentrations outside and inside the cell begin to coincide.
Thus, the presence of a stationary potential difference between the cytoplasm and the external environment in a resting cell is due to the existing concentration gradients for K +, Na + and Cl and different membrane permeability for these ions. The main role in the generation of MPP is played by the diffusion of potassium ions from the cell into the external solution. Along with this, the MPP is also determined by the sodium and chlorine equilibrium potentials and the contribution of each of them is determined by the relationship between the permeabilities of the cell plasma membrane for these ions.
All factors listed above constitute the so-called ionic componentMPP (Membrane Resting Potential). Since neither potassium nor sodium equilibrium potentials are equal to the MPP. the cell must absorb Na + and lose K +. The constancy of the concentrations of these ions in the cell is maintained due to the work of Na + K + -ATPase.
However, the role of this ion pump is not limited to maintaining sodium and potassium gradients. It is known that the sodium pump is electrogenic and during its functioning there is a pure flow of positive charges from the cell into the extracellular fluid, which causes an increase in the electronegativity of the cytoplasm in relation to the medium. The electrogenicity of the sodium pump was revealed in experiments on the giant neurons of the mollusc. The electrophoretic injection of Na + ions into the body of a single neuron caused hyperpolarization of the membrane, during which the IPP was significantly lower than the value of the potassium equilibrium potential. This hyperpolarization was weakened with a decrease in the temperature of the solution in which the cell was located, and was suppressed by the specific inhibitor of Na +, K + -ATPase ouabain.
It follows from the above that the MPP can be divided into two components - "Ionic"and "Metabolic".The first component depends on ion concentration gradients and membrane permeabilities for them. The second, "metabolic", is due to the active transport of sodium and potassium and has a double effect on MPP.On the one hand, the sodium pump maintains concentration gradients between the cytoplasm and the environment. On the other hand, being electrogenic, the sodium pump has a direct effect on MPP. Its contribution to the MPP value depends on the density of the "pumping" current (current per unit area of \u200b\u200bthe cell membrane surface) and the membrane resistance.
Membrane action potential
text_fields
text_fields
arrow_upward
If irritation is applied to a nerve or muscle above the excitation threshold, then the MPP of the nerve or muscle will rapidly decrease and for a short period of time (millisecond) the membrane will be recharged: its inner side will become positively charged relative to the outer one. it a short-term change in the MPP that occurs when the cell is excited, which on the oscilloscope screen has the form of a single peak, is called membrane action potential (MTD).
IVD in nerve and muscle tissues occurs when the absolute value of the MPP (membrane depolarization) decreases to a certain critical value, called generation thresholdMTD. In the giant nerve fibers of the squid, the IVD is - 60 mV. When the membrane is depolarized to -45 mV (the threshold of IVD generation), the IVD appears (Fig. 1.15).
Figure: 1.15 Action potential of the nerve fiber (A) and change in membrane conductivity for sodium and potassium ions (B).During the occurrence of IVD in the squid axon, the membrane resistance decreases 25 times, from 1000 to 40 Ohm.cm 2, while the electrical capacitance does not change. The indicated decrease in membrane resistance is due to an increase in the ionic permeability of the membrane upon excitation.
In terms of its amplitude (100-120 mV), the MTD (Membrane Action Potential) is 20-50 mV higher than the MPP (Resting Membrane Potential). In other words, the inner side of the membrane for a short time becomes positively charged with respect to the outer, - "overshoot" or charge reversion.
It follows from the Goldmann equation that only an increase in the membrane permeability for sodium ions can lead to such changes in the membrane potential. The E k value is always less than the MPP value; therefore, an increase in the membrane permeability for K + will increase the absolute MPP value. Sodium equilibrium potential has a plus sign, therefore, a sharp increase in the membrane permeability for these cations leads to a recharge of the membrane.
During IVD, the membrane permeability to sodium ions increases. Calculations have shown that if at rest the ratio of membrane permeability constants for K +, Na +, and SG is 1: 0.04: 0.45, then at MPD - P k: P Na: P \u003d 1: 20: 0.45 ... Consequently, in a state of excitation, the membrane of the nerve fiber not only loses its selective ionic permeability, but, on the contrary, from being selectively permeable at rest for potassium ions, it becomes selectively permeable for sodium ions. An increase in the sodium permeability of the membrane is associated with the opening of voltage-dependent sodium channels.
The mechanism that provides the opening and closing of ion channels is called channel gate.It is customary to distinguish activation(m) and inactivating(h) gate. The ion channel can be in three basic states: closed (m-gate is closed; h-open), open (m- and h-gate are open) and inactivated (m-gate is open, h-gate is closed) (Figure 1.16).
Figure: 1.16 Scheme of the position of the activation (m) and inactivation (h) gates of sodium channels, corresponding to the closed (rest, A), open (activation, B) and inactivated (C) states.
Depolarization of the membrane, caused by an irritating stimulus, for example, an electric current, opens the m-gate of sodium channels (transition from state A to B) and provides an inward flow of positive charges - sodium ions. This leads to further membrane depolarization, which, in turn, increases the number of open sodium channels and, therefore, increases the sodium permeability of the membrane. A “regenerative” depolarization of the membrane occurs, as a result of which the potential of the inner side of the membrane tends to reach the value of the sodium equilibrium potential.
The reason for the termination of the growth of IVD (Membrane Action Potential) and repolarization of the cell membrane is:
a) Increased membrane depolarization, i.e. when E m - »E Na, as a result of which the electrochemical gradient for sodium ions decreases, equal to E m -\u003e E Na. In other words, the force that "pushes" sodium into the cell decreases;
b) Depolarization of the membrane gives rise to the process of inactivation of sodium channels (closing of the h-gate; state of the B channel), which inhibits the growth of sodium permeability of the membrane and leads to its decrease;
in) Depolarization of the membrane increases its permeability to potassium ions. The outgoing potassium current tends to shift the membrane potential towards the potassium equilibrium potential.
A decrease in the electrochemical potential for sodium ions and inactivation of sodium channels decreases the amount of incoming sodium current. At a certain moment in time, the value of the input sodium current is compared with the increased output current - the growth of the MPD stops. When the total outgoing current exceeds the incoming, membrane repolarization begins, which also has a regenerative character. The incipient repolarization leads to the closing of the activation gate (m), which decreases the sodium permeability of the membrane, accelerates repolarization, and the latter increases the number of closed channels, etc.
The IVD repolarization phase in some cells (for example, in cardiomyocytes and a number of smooth muscle cells) can slow down, forming plateauAP, due to complex changes in time of the incoming and outgoing currents through the membrane. Hyperpolarization and / or depolarization of the membrane may occur in the aftereffect of the IVD. These are the so-called trace potentials.Trace hyperpolarization has a twofold nature: ionicand metabolicforge.The first is associated with the fact that the potassium permeability in the nerve fiber of the membrane remains for some time (tens and even hundreds of milliseconds) increased after IVD generation and shifts the membrane potential towards the potassium equilibrium potential. Trace hyperpolarization after rhythmic stimulation of cells is associated mainly with the activation of an electrogenic sodium pump due to the accumulation of sodium ions in the cell.
The reason for the depolarization that develops after the generation of the IVD (Membrane Action Potential) is the accumulation of potassium ions at the outer surface of the membrane. The latter, as follows from the Goldmann equation, leads to an increase in the MPP (Resting Membrane Potential).
An important property of the nerve fiber is associated with the inactivation of sodium channels, calledrefractoriness .
During absofiercerefractory period the nerve fiber completely loses its ability to be excited by the action of an irritant of any strength.
Relativerefractoriness, following the absolute one, is characterized by a higher threshold of IVD (Membrane Action Potential).
The concept of membrane processes occurring during excitation of a nerve fiber serves as a basis for understanding the phenomenon accommodation.At the heart of tissue accommodation at a low steepness of the increase in the irritating current is an increase in the excitation threshold, which is ahead of the slow depolarization of the membrane. The increase in the excitation threshold is almost entirely determined by the inactivation of sodium channels. The role of increasing the potassium permeability of the membrane in the development of accommodation is that it leads to a drop in membrane resistance. Due to the decrease in resistance, the rate of membrane depolarization becomes even slower. The rate of accommodation is the higher, the greater the number of sodium channels at the resting potential is in an inactivated state, the higher the rate of development of inactivation, and the higher the potassium permeability of the membrane.
Conducting arousal
text_fields
text_fields
arrow_upward
Conduction of excitation along the nerve fiber is carried out due to local currents between the excited and resting sections of the membrane. The sequence of events in this case is presented as follows.
When a point stimulation is applied to a nerve fiber, an action potential arises in the corresponding section of the membrane. The inner side of the membrane at this point is positively charged with respect to the neighboring, resting one. A current arises between fiber points at different potentials (local current),directed from excited (sign (+) on the inner side of the membrane) to unexcited (sign (-) on the inner side of the membrane) to the fiber section. This current has a depolarizing effect on the fiber membrane in the resting area, and when the critical level of membrane depolarization is reached in this area, an IVD (Membrane Action Potential) appears. This process sequentially spreads to all parts of the nerve fiber.
In some cells (neurons, smooth muscles), IVD is not of a sodium nature, but is due to the input of Ca 2+ ions through voltage-dependent calcium channels. In cardiomyocytes, IVD generation is associated with incoming sodium and sodium-calcium currents.
In this topic, two cations will be considered - sodium (Na) and potassium (K). Speaking about anions, let us take into account that a certain amount of anions is located at the outer and inner sides of the cell membrane.
The shape of the cell depends on which tissue it belongs to. According to its form cells can be:
· Cylindrical and cubic (skin cells);
Disciform (erythrocytes);
Spherical (eggs);
Fusiform (smooth muscle);
Stellate and pyramidal (nerve cells);
· Not having a permanent form - amoeba (leukocytes).
The cell has a number properties: it feeds, grows, multiplies, restores, adapts to its environment, exchanges energy and substances with the environment, performs its inherent functions (depending on which tissue a given cell belongs to). In addition, the cell has excitability.
Excitability – it is the ability of a cell to move from a state of rest to a state of activity in response to stimuli.
Irritations can come from the external environment or occur inside the cell. Stimuli that cause excitement can be: electrical, chemical, mechanical, temperature and other stimuli.
The cell can be in two basic states - at rest and in arousal. The rest and excitement of the cell is otherwise called - resting membrane potential and membrane action potential.
When the cell does not experience any stimulation, it is at rest. The rest of the cell is called differently resting membrane potential (MPP).
At rest, the inner surface of its membrane is charged negatively, and the outer surface is positively charged. This is explained by the fact that inside the cell there are many anions and few cations, while behind the cell, on the contrary, cations prevail.
Since the cell contains electric chargesthen the electricity they create can be measured. The value of the resting membrane potential is: - 70 mV, (minus 70, since there is a negative charge inside the cell). This value is arbitrary, since each cell may have its own value of the resting potential.
At rest, the pores of the membrane are open for potassium ions and closed for sodium ions. This means that potassium ions can easily enter and leave the cell. Sodium ions cannot enter the cell, since the pores of the membrane are closed for them. But a small number of sodium ions penetrate into the cell, because they are attracted by a large number of anions located on the inner surface of the membrane (opposite charges are attracted). This movement of ions is passive , since it does not require energy consumption.
For normal cell activity, the value of its MPP must remain at a constant level. However, the movement of sodium and potassium ions across the membrane causes fluctuations in this value, which can lead to a decrease or increase in the value: - 70 mV.
In order for the MPP value to remain relatively constant, the so-called sodium - potassium pump . Its function lies in the fact that it removes sodium ions from the cell, and pumps potassium ions into the cell. It is a certain ratio of sodium and potassium ions in the cell and behind the cell that creates the required MPP value. Pump operation is active mechanism , because it requires energy.
The energy source in the cell is ATP. ATP gives energy only when it is split into a simpler acid - ADP, with the obligatory participation of the enzyme ATP-ase in the reaction:
ATP + enzyme ATP-ase ADP + energy
All living cells have the ability, under the influence of stimuli, to pass from a state of physiological rest to a state of activity or excitement.
Excitation is a complex of active electrical, chemical and functional changes in excitable tissues (nervous, muscular or glandular), with which the tissue responds to external influences. An important role in excitation is played by electrical processes that ensure the conduction of excitation through the nerve fibers and bring the tissues into an active (working) state.
Membrane potential
Living cells have an important property: the inner surface of the cell is always charged negatively in relation to its outer side. Between the outer surface of the cell, charged electropositively with respect to the protoplasm, and the inner side of the cell membrane, there is a potential difference that ranges from 60 to 70 mV. According to the data of PG Kostyuk (2001), in a nerve cell this difference ranges from 30 to 70 mV. The potential difference between the outer and inner sides of the cell membrane is called membrane potential,or resting potential (fig. 2.1).
The resting membrane potential is present on the membrane as long as the cell is alive and disappears with cell death. L. Galvani back in 1794 showed that if you damage a nerve or muscle, making cross section and by applying electrodes connected to the galvanometer to the damaged part and to the place of damage, the galvanometer will show the current that always flows from the undamaged part of the tissue to the site of the incision. He called this flow the quiescent current. By their physiological essence, the rest current and the resting membrane potential are one and the same. The potential difference measured in this experiment is 30-50 mV, since when tissue is damaged, part of the current is shunted in the intercellular space and the fluid surrounding the structure under study. The potential difference can be calculated using the Nernst formula:
where R is the gas constant, T is the absolute temperature, F is the Faraday number, [K] ext. and [K] bunk. - the concentration of potassium inside and outside the cell.

Figure: 2.1.
The reason for the emergence of the resting potential is common for all cells. There is an uneven distribution of ions (ionic asymmetry) between the protoplasm of the cell and the extracellular environment. The composition of human blood in terms of salt balance resembles the composition of ocean water. The extracellular environment in the central nervous system also contains a lot of sodium chloride. The ionic composition of the cytoplasm of cells is poorer. Inside cells, there are 8-10 times less Na + ions and 50 times less C ions! " K + in the cytoplasm are organic anions, in particular anions of aspartic, histamine and other amino acids. Such an asymmetry is a violation of thermodynamic equilibrium. In order to restore it, potassium ions must gradually leave the cell, and sodium ions - tend to it. However, this is not going on.
The first obstacle to equalizing the difference in ion concentrations is the plasma membrane of the cell. It consists of a double layer of phospholipid molecules covered from the inside with a layer of protein molecules, and from the outside with a layer of carbohydrates (mucopolysaccharides). Some of the cellular proteins are embedded directly into the lipid bilayer. These are internal proteins.
Membrane proteins of all cells are divided into five classes: pumps, channels, receptors, enzymes and structural proteins. Pumps serve to move ions and molecules against concentration gradients using metabolic energy. Protein channels, or pores, provide selective permeability (diffusion) through the membrane of the corresponding size of ions and molecules. Receptor proteins with high specificity, recognize and bind, attaching to the membrane, many types of molecules necessary for the vital activity of the cell at any given time. Enzymes accelerate the flow chemical reactions at the membrane surface. Structural proteins ensure the connection of cells into organs and maintenance of the subcellular structure.
All of these proteins are specific, but not strictly. Under certain conditions, a particular protein can be both a pump, an enzyme, and a receptor. Through the channels of the membrane, water molecules, as well as ions corresponding to the pore sizes, enter and leave the cell. The membrane permeability for different cations is not the same and changes under different functional states of the tissue. At rest, the membrane is 25 times more permeable to potassium ions than to sodium ions, and when excited, sodium permeability is about 20 times higher than potassium. At rest, equal concentrations of potassium in the cytoplasm and sodium in the extracellular environment should provide an equal amount of positive charges on both sides of the membrane. But since the permeability for potassium ions is 25 times higher, then potassium, leaving the cell, makes its surface more and more positively charged with respect to the inner side of the membrane, around which negatively charged molecules of aspartic, histamine and other molecules that are too large for the pores of the membrane accumulate. amino acids that "released" potassium outside the cell, but "do not allow" it to go far due to its negative charge. Negative charges accumulate on the inside of the membrane, and positive charges on the outside. A potential difference arises. The diffuse current of sodium ions into the protoplasm from the extracellular fluid keeps this difference at the level of 60-70 mV, preventing it from increasing. The diffuse current of sodium ions at rest is 25 times weaker than the counter current of potassium ions. Sodium ions, penetrating into the cell, reduce the value of the resting potential, allowing it to be kept at a certain level. Thus, the value of the resting potential of muscle and nerve cells, as well as nerve fibers, is determined by the ratio of the number of positively charged potassium ions diffusing per unit time from the cell to the outside and positively charged sodium ions diffusing through the membrane in the opposite direction. The higher this ratio, the greater the value of the resting potential, and vice versa.
The second obstacle keeping the potential difference at a certain level is the sodium-potassium pump (Fig. 2.2). It is called sodium-potassium or ionic, since it actively removes (pumps out) sodium ions penetrating into it from the protoplasm and introduces (injects) potassium ions into it. The source of energy for the operation of the ion pump is the cleavage of ATP (adenosine triphosphate), which occurs under the influence of the enzyme adenosine triphosphatase, localized in the cell membrane and activated by the same ions, i.e., potassium and sodium (sodium-potassium-dependent ATP-ase).

Figure: 2.2.
It is a large protein that is larger than the thickness of the cell membrane. The molecule of this protein, penetrating the membrane through and through, binds mainly sodium and ATP on the inner side, and potassium and various inhibitors of the glycoside type on the outer side. This produces a membrane current. Due to this current, an appropriate direction of ion transport is ensured. Ion transfer occurs in three stages. First, an ion combines with a carrier molecule to form a carrier ion complex. This complex then passes through the membrane or transfers charge through it. Finally, the ion is freed from the carrier on the opposite side of the membrane. At the same time, a similar process takes place, transferring ions in the opposite direction. If the pump transfers one sodium ion to one potassium ion, then it simply maintains the concentration gradient on both sides of the membrane, but does not contribute to the creation of the membrane potential. To make this contribution, the ion pump must transfer sodium and potassium in a ratio of 3: 2, that is, for 2 potassium ions entering the cell, it must remove 3 sodium ions from the cell. Operating at maximum load, each pump is capable of pumping about 130 potassium ions and 200 sodium ions per second across the membrane. This is the ultimate speed. In real life, each pump is adjusted according to the needs of the cage. Most neurons have between 100 and 200 ion pumps per square micron of membrane surface. Consequently, the membrane of any nerve cell contains 1 million ion pumps capable of moving up to 200 million sodium ions per second.
Thus, the membrane potential (resting potential) is created as a result of both passive and active mechanisms. The degree of participation of certain mechanisms in different cells is not the same, from which it follows that the membrane potential may be different in different structures. The activity of the pumps may depend on the diameter of the nerve fibers: the thinner the fiber, the higher the ratio of the surface size to the volume of the cytoplasm, respectively, and the activity of the pumps required to maintain the difference in ion concentrations on the surface and inside the fiber must be greater. In other words, the membrane potential may depend on the structure of the nervous tissue, and hence on its functional purpose. The electrical polarization of the membrane is the main condition for the excitability of the cell. This is her constant readiness for action. This is a store of potential energy of the cell, which it can use in case the nervous system needs its immediate reaction.