Most foods contain proteins, fats and carbohydrates, which, in the presence of water, are a good breeding ground for microorganisms. While multiplying, they decompose the constituent parts of food products, forming decay products (intermediate and final). This is due to the enzymatic activity of microorganisms, many of them. which produce strong proteolytic, amylolytic and lipolytic enzymes. Their use in various areas of the national economy is based on the ability of microbes to secrete certain enzymes. It has long been known and widely used, for example, in Food Industry and everyday life the ability of yeast to decompose sugar. Release enzymes amylase, maltase and sucrose, as well as prothiolytic enzymes, yeast breaks down carbohydrates and partially proteins, forming alcohol and carbon dioxide. This property is used in the wine, brewing and bakery industries. Due to the formation of carbon dioxide during the fermentation of the dough, it loosens, which makes it possible to obtain porous ("fluffy") bread products during baking. The taste and digestibility of bread is improved as a result of the use of yeast. Some microbes are widely used in the manufacture of lactic acid products, causing lactic acid fermentation, in which milk sugar decomposes and lactic acid is formed.
This ability is possessed by lactic acid streptococcus, Bulgarian and acidophilus sticks. By selecting cultures of lactic acid microbes, you can torment a variety of different types lactic acid products with high taste and dietary properties. Cooking sauerkraut and pickled cucumbers is also based on the ability of microbes to induce lactic acid fermentation. In the preparation of salted herring, kilka, anchovies, the property of microbes is used to induce proteolytic changes in tissues - to break down protein. Due to the partial splitting of protein molecules and changes in the physicochemical properties of products under the influence of these microbes, a specific aroma and taste are created.
Not only are the beneficial properties of microbes known, but also their negative effect on food. Many microorganisms, causing the decomposition of the constituent parts of a food product, do not improve, but worsen its quality. These microorganisms primarily include putrefactive: Bact. Proteus vulgaries, Bact. Cloacae, Bact. Putrificus, sporogenes, etc. The growth and reproduction of these microbes is accompanied by the decomposition of protein substances and the accumulation of decay products, many of which have an unpleasant taste or have a strong unpleasant odor. These include such organic substances as indole, skatole, cadaverine, histamine, gases - hydrogen sulfide, ammonia, phosphine, methylamine.
Many methods of sanitary examination of food products are based on the determination of intermediate decomposition products. As a result of putrefactive decay, the surface of food products with a dense consistency becomes slimy, becomes sticky. Due to a complex of changes during decay, food products lose their original organoleptic properties and become of poor quality.
When rotting in food, microbes pathogenic for humans can also multiply, for example, salmonella, botulinus bacillus, since pathogenic microorganisms are especially good at using for their nutrition and assimilating the products of partial breakdown of protein. In this regard, food products with the phenomena of putrefactive decomposition, if consumed, pose a great danger in relation to food poisoning. Workers in the food industry, catering and trade are obliged to comply with the necessary conditions for the protection of products from microbial decomposition. The conditions favorable for the reproduction of putrefactive microbes are heat, the presence of protein and moisture in the product, and low acidity. The high protein content in the aquatic environment is an excellent breeding ground for microbes. Products such as meat, milk, fish, eggs, and boiled sausages are especially quickly exposed to putrefactive decomposition.
In conditions of elevated temperatures, the reproduction of microbes is significantly accelerated. Along with the growth of microbes and the enhancement of their enzymatic activity, the enzymes found in the tissues themselves are activated. These enzymes also break down proteins, fats, and carbohydrates to form the same breakdown products as when rotting. The greatest reproduction of putrefactive microbes and the action of enzymes occur at a temperature of 20-25 ° C (up to 40-45 ° C). Low temperatures and low humidity, on the contrary, create unfavorable conditions for the growth of bacteria.
Consequently, the main condition that is widely used in the practice of food enterprises in order to preserve food is the use of a low temperature (storage of perishable food in special refrigerated cabinets or refrigerators). However, it should be remembered that the cold does not cause the death of microbes, but only delays or stops their vital activity and that under favorable conditions they can continue to adversely affect the quality of food. In addition, there are some types of bacteria that can multiply in conditions of low temperatures, even close to 0 degrees. (eg Bact. Fluorescens) as well as numerous molds.
In addition to cooling, drying or adding substances that increase the concentration of hydrogen ions (pickling), as well as other methods of preservation, which create unfavorable conditions for the development of microbes, are used to protect products from the multiplication of microbes in them. Under the influence of microbes during storage, the properties of fat-containing products also change: lard, butter, chocolate. In this case, microbes such as Bact play an important role. fluorescens. Bact. pyocyaneum), as well as some mushrooms (Penicillium aspergillus). These microbes secrete an enzyme called lipase, which breaks down fat into its constituent parts - glycerin and fatty acids. The accumulation of free fatty acids in fat increases its acidity.
However, the properties of fats change mainly under the influence of physical factors - oxygen in the air and light. Under the influence of atmospheric oxygen, fat is oxidized. It accumulates aldehydes, ketones, oxidized acids, which lead to rancidity or salting of foods containing fat. When burned out, the taste of the product becomes bitter; when tasted, foods containing fat taste like a stearicum candle. Sunlight intensifies oxidation tenfold. The quality of food is highly dependent on the humidity of the ambient air. With high humidity, some foods (dried fruits and vegetables, sugar, salt, confectionery, crackers, flour) greedily absorb moisture from the air and become humidified, which contributes to mold.
In addition, the nutritional value of moisturized foods is reduced because moist foods contain fewer nutrients at the same weight. In excessively dry rooms, due to increased evaporation, food shrinks and their weight decreases. When vegetables are dried, along with the deterioration of the presentation, the content of vitamins in them decreases. The combination of high humidity and high temperature stimulates the processes of tissue respiration and growth in foods such as potatoes, beets, carrots, onions and other root crops.
Their germination leads to an irrational expenditure of reserves accumulated in plants (carbohydrates, vitamins, mineral elements) and a decrease in the nutritional value of these products under these conditions. The quality of food products can be reduced by careless handling during transportation, sale, storage. They can become dirty, change their original shape, acquire an unpleasant taste or odor. Mechanical impurities (earth, sand, glass) or poisonous substances (salts of heavy metals - lead, copper, zinc) can get into food products from the outside (earth, sand, glass).
The admixture of earth and sand to foods not only impairs their taste, but also poses an epidemiological danger, since spores of B. botulinus, eggs of some helminths, etc. can enter the human body with food. Contamination of food with B. botulinu spores during their germination, reproduction and toxin formation often leads to poisoning - botulism. The presence of helminth eggs in food products can cause helminthic diseases among people if sanitary and hygienic rules are not followed during the processing of contaminated products. Therefore, during storage, transportation and sale, conditions must be strictly observed that contribute to the preservation of the original quality of the products.
Foods infected with pathogenic microbes - dysentery, typhoid bacilli, paratyphoid pathogens, etc., entering the human body, can cause severe infectious diseases - dysentery, typhoid fever, paratyphoid fever. Certain microbes can cause foodborne illness. Such microbes include salmonella, pathogenic serotypes of Escherichia coli, causative agents of botulism, an enterotoxic strain of staphylococcus.
The causative agent of botulism B. botulinus and the enterotoxic strain of staphylococcus, when multiplying in products, are capable of forming poisons - exotoxins. The use of such products causes intoxication of the human body. Pathogenic staphylococci are widespread in nature. They can get into food from the hands, especially in case of pustular diseases, from the upper respiratory tract (catarrh, tonsillitis, dental disease), in the unsanitary condition of the premises where food is prepared, from animals sick with mastitis.
Foods contaminated with pathogens of infectious diseases and food poisoning are especially dangerous for catering enterprises and organized groups (kindergartens, pioneer camps, etc.), since in this case, diseases become widespread. An example is food poisoning in one of these groups, where 186 children fell ill as a result of consuming vinaigrette, for which potatoes and beets were boiled and peeled the night before, chopped and left until morning without sufficient cooling. In the morning, onions and cabbage were added to the potatoes and beets. The vinaigrette was given to the children for breakfast. During the investigation of this poisoning, pathogenic Staphylococcus aureus was isolated from the vinaigrette, as well as from the throat of two cooks taking part in the cleaning of boiled potatoes and beets, giving all the characteristic reactions and samples to it.
Proteins are decomposed by actinomycetes either to final products (hydrogen sulfide, ammonia and water), or to the formation of intermediate substances (peptones, amino acids). The intensity of protein degradation depends on the conditions of aeration, the composition of the nutrient medium, temperature and other factors. [...]
The decomposition of nitrogen-containing substances (proteins) takes place in two stages. At the first stage, under the influence of aerobic and anaerobic microorganisms, proteins are broken down with the release of the nitrogen contained in them in the form of MNs (ammonification stage) and the formation of peptones (products of the primary breakdown of proteins), and then amino acids. Subsequent oxidative and reductive deamination and decarboxylation lead to the complete breakdown of peptones and amino acids. The duration of the first stage is from one to several years. In the second stage, NH3 is first oxidized to H102, and then to NH3O3. The final return of nitrogen to the atmosphere occurs under the action of bacteria - denitrifiers, which decompose molecular nitrogen nitrates. The duration of the mineralization period is 30-40 years or more. [...]
Decomposition of sulfur containing compounds. Sulfur is found in some proteins. During the hydrolytic breakdown of proteins, it is reduced to hydrogen sulfide, which is a toxic compound for many groups of microorganisms. But in water bodies and soil, sulfur bacteria are found, oxidizing reduced sulfur compounds to free sulfur and sulfates. These bacteria live in high concentrations of hydrogen sulfide in the environment. Hydrogen sulfide for them serves as a source of energy for synthesis organic matter.[ ...]
Decomposition includes both abiotic and biotic processes. However, usually dead plants and animals are decomposed by heterotrophic microorganisms and saprophages. This decomposition is the way bacteria and fungi obtain food for themselves. Decomposition, therefore, occurs through energetic transformations in and between organisms. This process is absolutely necessary for life, since without it all nutrients would be bound in dead bodies and no new life could not arise. In bacterial cells and mycelium of fungi, there are sets of enzymes necessary for the implementation of specific chemical reactions... These enzymes are released into dead matter; some of the products of its decomposition are absorbed by decomposing organisms, for which they serve as food, others remain in the environment; in addition, some products are removed from the cells. Not a single species of saprotroph can carry out the complete decomposition of a dead body. However, the heterotrophic population of the biosphere consists of a large number of species, which, acting together, produce complete decomposition. Different parts of plants and animals are destroyed at different rates. Fats, sugars and proteins decompose quickly, while plant cellulose and lignin, chitin, animal hair and bones are destroyed very slowly. Note that about 25% of the dry weight of the herbs decomposed within a month, while the remaining 75% decomposed more slowly. After 10 months. there still remained 40% of the original mass of herbs. The remains of the crabs had completely disappeared by this time. [...]
During the decomposition of proteins, ammonia and its derivatives are also formed, which also enter the air and water of the ocean. In the biosphere, as a result of nitrification - the oxidation of ammonia and other nitrogen-containing organic compounds with the participation of bacteria - various nitrogen oxides are formed, which are the basis for the formation of nitric acid. Nitric acid combines with metals to form salts. As a result of the activity of denitrophying bacteria, nitric acid salts are reduced to nitrous acid and then to free nitrogen. [...]
Anaerobic degradation of proteins is caused by spore-forming rods: Bacillus putrificus, Bacillus sporogenes. The decomposition of protein compounds is also caused by the facultative anaerobes Proteus vulgaris, Bacteria coli. The degree and intensity of the decomposition of protein compounds depends on chemical structure protein and the type of microorganisms. Amino acids formed during the breakdown of proteins under anaerobic conditions undergo reductive deamination with the formation of saturated organic acids and ammonia. Organic acids can decompose to form methane and carbon dioxide. The products of ammonification under anaerobic conditions will be methane, ammonia and carbon dioxide. [...]
[ ...]
Occurs in the decomposition of alkaloids and proteins. [...]
AMMONIFICATION - the process of decomposition by microorganisms of nitrogen-containing organic compounds (proteins, nucleic acids, etc.) with the release of ammonia. ENVIRONMENTAL AMPLITUDE [lat. amplitude - value] - the limits of adaptability of a species or community to changing environmental conditions. [...]
The ammonia formed during the decomposition of proteins and urea in the form of ammonium salts is assimilated by plants or undergoes further microbiological transformations. [...]
The most stable decomposition products are humic substances (humus), which, as already emphasized, are an essential component of ecosystems. It is convenient to distinguish three stages of decomposition: 1) grinding detritus by physical and biological impact; 2) the relatively rapid formation of humus and the release of soluble organic substances by saprotrophs; 3) slow humus mineralization. The slow decomposition of humus is one of the factors responsible for the delay in decomposition in comparison with the production and accumulation of oxygen; the significance of the last two processes has already been mentioned. Humus usually appears as a dark, often yellowish-brown, amorphous or colloidal substance. According to M.M. Kononova (1961), physical properties and chemical structure humus differ little in geographically distant or biologically different ecosystems. However, it is very difficult to characterize the chemical substances of humus, and this is not surprising given the huge variety of organic substances from which it comes. In general, humic substances are condensation products of aromatic compounds (phenols) with decomposition products of proteins and polysaccharides. The molecular structure model of humus is shown on page 475. It is a phenol benzene ring with side chains; such a structure determines the resistance of humic substances to microbial decomposition. The cleavage of compounds obviously requires special enzymes of the deoxygenase type (Gibson, 1968), which are often absent in common soil and water saprotrophs. Ironically, many toxic products that humans introduce into the environment - herbicides, pesticides, industrial wastewater - are derivatives of benzene and pose a serious hazard due to their resistance to degradation. [...]
Ammonia is formed mainly during the decomposition of biogenic nitrogen-containing compounds - proteins and urea. The most probable value of the flux 1> W3 from all terrestrial sources into the atmosphere is 70-100 MtN / year. Anthropogenic emission of ammonia is only about 4 Mt K / yr. [...]
This can be explained by the lower ratio of proteins and carbohydrates to the amount of fats in the wastewater sludge of the meat processing plant in comparison with the domestic wastewater sludge; As you know, the main material for building the body of microorganisms involved in the decomposition of fats are proteins in conjunction with carbohydrates, and carbohydrates are the energy material for their vital activity. Therefore, the ratio of fermentable components affects the decomposition of organic substances. [...]
V.S.Butkevich's research contributed a lot to revealing the essence of the decomposition of organic nitrogenous compounds. He was able to show that the accumulation of ammonia during ammonification processes is strictly coordinated with the presence of carbohydrates in the medium. If there are no carbohydrates in the medium, then microorganisms intensively use protein substances as a material for respiration, and the nitrogen of oxidized amino acids accumulates in the form of ammonia. If carbohydrates are available, then protein substances are used to a lesser extent and the accumulation of ammonia is greatly reduced, and sometimes does not occur at all. These patterns are very important in the fermentation of sewage sludge. By the presence of ammonium salts in the sludge liquid, one can judge which substances undergo decomposition: proteins or carbohydrates. [...]
The decomposition of the main organic components of the sediment - protein, fats, carbohydrates - occurs with varying intensity, depending on the predominant form of certain microorganisms. So, for example, septic tanks are characterized by an environment that creates conditions for the development of anaerobic putrefactive bacteria of the first stage (phase) of decomposition of organic matter. [...]
Almost all the nitrogen taken by the plant from the soil is part of the plant protein, which, when decaying (decaying), breaks off nitrogen in the form of ammonia, and it can be felt in the stable when horse manure decomposes (horse manure is characterized by especially vigorous decomposition, which is why it is used for heating greenhouses). [...]
Nitrogen is one of the most essential nutrients for plants. It is a part of proteins, chlorophyll, and many other organic substances in plants. The bulk of the aza is concentrated in the organic matter of the soil, and firstly in humus. Nitrogen is available to plants mainly in the fs of mineral compounds - ammonia and nitrates, which are formed during the decomposition of organic matter by special microorganisms. Therefore, it is necessary to replenish the reserves of soil nitrogen from other sources . [...]
Organic substances contained in the soil include substances formed during the decomposition of proteins, fats, carbohydrates, including: resins, fiber, essential oils... For the processes of decomposition of organic matter, the content of organisms - destructors (bacteria, protozoa) is important. One hectare of soil can contain from 1000 to 7000 kg of various bacteria, 350-1000 kg of worms, up to 1000 kg of arthropods, from 100 to 1000 kg of microscopic fungi. These microorganisms are found throughout the entire soil thickness, which can reach several meters. Invertebrates mainly live in the upper layers. Similarly, the root system of plants is located mainly at depths of several meters (with the exception of some, for example, camel thorn, the roots of which penetrate 15 m deep). [...]
Waste water smell populated areas, which is a mixture of feces odor with decomposition odors of fats, proteins, soap, etc., is quite characteristic. It depends on the decomposition of household wastewater and on which processes prevail in the water - oxidative or reducing. Some wastewater from food processing plants may also have a similar odor. Waste water from thermal processing of coal smells like phenols, tar, hydrogen sulfide; Wastewater from the chemical industry has characteristic odors depending on the type of production, for example, the smell of organic compounds: carbon disulfide, esters and ethers, alcohols, organic acids, nitrogen-containing compounds, mercaptans, acetylene, etc. [...]
The polysaprobic zone is typical for freshly polluted water, where the initial stages of decomposition of organic compounds take place. Polysaprobic waters contain a large amount of organic matter, primarily proteins and carbohydrates. When these substances decompose in large quantities, carbon dioxide, hydrogen sulfide, methane are released. Water is poor in oxygen, therefore, chemical processes are of a reducing nature. Pronounced unfavorable environmental conditions lead to a limitation of the number of species in the plant and animal population of the reservoir. The main inhabitants are bacteria, the number of which reaches hundreds of millions in 1 ml of water. There are a lot of sulfur bacteria and ciliates. All inhabitants of the polysaprobic zone, according to the way of feeding, belong to coyasuyens (consumers), or otherwise heterotrophs. They need ready-made organic matter. Producers (producers), ie autotrophs, which include green plants that create organic matter from mineral compounds, are completely absent here. [...]
The composition of organic substances is diverse and includes components formed at different stages of decomposition of complex carbohydrates, proteins, fats and carbohydrates; soil organic matter contains lignin, fiber, essential oils, resins, tannins. A certain role in the creation of humus is played by the soil fauna - worms and specific soil microflora. In general, the soil is enriched with amino acids and other organic compounds. [...]
The literature indicates that humic substances arise in natural conditions as decomposition products of proteins, cellulose and lignin. They are divided into humic acids and insoluble lignin. In this work, only humic acids are considered, the salts of which are soluble in water and capable of leaching. [...]
Other physiological groups of anaerobes are involved in the cycle of nitrogen-containing substances: they decompose proteins, amino acids, purines (proteolytic, purinolytic bacteria). Many are able to actively fix atmospheric nitrogen, converting it into an organic form. These anaerobes increase soil fertility. The number of cells of proteolytic and saccharolytic anaerobes in 1 g of fertile soils reaches even millions. Of particular importance are those groups of microorganisms that are involved in the decomposition of hard-to-reach forms of organic compounds, such as pectin and cellulose. It is these substances that make up a large proportion of plant residues and are the main source of carbon for soil microorganisms. [...]
In the process of vital activity, many bacteria can acidify or alkalize the environment. For example, during the decomposition of urea or proteins, ammonia is formed, and when salts of organic acids are consumed, alkali metal cations accumulate in the medium. [...]
Oxidation of protein compounds occurs to the end with the formation of ammonia, carbon dioxide, water. If the proteins contain sulfur, then mercaptans (thioalcohols) are also formed as intermediate compounds, and when completely decomposed, hydrogen sulfide is formed. The most common aerobic protein breakdown agents: Bacterium fluorescens, Bacillus subtilis, Bacillus mycoides. In addition, the decomposition of protein compounds can be caused by actinomycetes and many fungi. Nucleoproteins containing nucleic acids bound to amino acid residues decompose with the formation of carbohydrates - ribose and deoxyribose, nitrogenous organic bases and phosphoric acid.[ ...]
Sulfur dioxide is released into the atmosphere during the combustion of fossil fuels (coal, oil, gasoline, gas) due to the decomposition of sulfur-containing proteins, as well as from enterprises processing sulfur ores. Motor vehicles are a powerful source of sulfur dioxide emission in cities. [...]
Nitrogen-containing substances (ammonium salts, nitrites and nitrates) are formed in water mainly as a result of the decomposition of protein compounds that enter the reservoir with domestic and industrial waste water. Less common in water is found ammonia of mineral origin, formed as a result of the reduction of organic nitrogenous compounds. If the reason for the formation of ammonia is the decay of proteins, then such waters are not suitable for drinking. [...]
The first two groups use more easily degradable organic substances such as sugars, amino acids and simple proteins. Then cellulose bacteria begin their "work" on more stable compounds, while actinomycetes are directly related to humus. A possible model of the structure of the humic acid molecule is shown below. [...]
Wastewater sludge and concentrated industrial wastewater with a MIC of more than 5 g / l are biodegradable under anaerobic conditions. It can occur in septic tanks, which are a sump through which waste fluid slowly flows. In a two-tier sedimentation tank, the sludge is separated from the passing waste liquid, its decomposition is carried out in a sludge chamber. On the treatment facilities of high productivity, sewage sludge is released in primary sedimentation tanks and, together with excess activated sludge, is fermented in digesters. The intensity and depth of sediment decomposition are primarily determined by its composition, which fluctuates according to the ratio of the content of the main organic components (carbohydrates / proteins, fat-like compounds) and inorganic substances. Typically, urban wastewater sludge contains 70-80% organic matter. So, the approximate composition of the sediment (%): proteins 24, carbohydrates 23, fat-like substances up to 30. Most often, acidic fermentation of the sediment produces acetic, butyric, propionic acids. The resulting gases contain carbon dioxide, methane, hydrogen, hydrogen sulfide. The aqueous phase has an acidic reaction of the medium (pHС5), does not possess buffering properties, has a strong unpleasant odor. [...]
With domestic and industrial wastewater, including wastewater from industrial sites, proteins, fats, oils, oil and oil products, dyes, resins, tannins, enter water bodies, detergents and many other pollution. Fertilizers and pesticides are washed out from the fields - means of combating agricultural pests. Therefore, in the waters of open sources of water supply, in different concentrations, virtually any chemical elements, including such unhealthy ones as lead, zinc, tin, chromium, copper. Without intending to give a complete overview of the composition of contaminants entering with wastewater, and assuming that the properties of biological impurities are considered in sufficient detail in the previous section of this chapter, we will dwell only on some types of contaminants, the hallmarks of which are: last years; toxic properties; difficult separation in wastewater treatment; slow oxidation and decomposition in open water bodies; interfering effect on water purification processes, including coagulation; the ability “to be indicators of the depth of water purification from individual [elements. [...]
The formation of humic substances occurs with the participation of two types of processes. Processes of the first type provide partial decomposition (splitting) of dead organic matter to simpler compounds: proteins are broken down into amino acids, carbohydrates - into simple sugars, the breakdown of lignin has not been studied enough. As a result of processes of the second type, condensation of aromatic compounds of the phenolic type (decomposition products of lignin and cellulose) with amino acids (decomposition products of microorganisms) occurs. As a result, a system of organic high-molecular acids arises, capable of further polymerization. In the process of humus formation and maintenance of its composition, an important role is played by heterotrophic and autotrophic microorganisms, the geochemical activity of which was discussed earlier. [...]
Organic composition. It is formed from compounds found in large quantities in plant and animal residues. These are proteins, carbohydrates, organic acids, fats, lignin, tannins, etc., which together make up 10-15% of the total mass of organic matter in the soil. When organic substances decompose, the nitrogen contained in them passes into forms available to plants. Organic substances play an important role in soil formation, determine the absorption capacity of soils, affect the structure of the upper horizons of the soil and its physical properties. [...]
A significant part of the nitrogen of humic acids goes into solution with weaker hydrolysis (S. S. Dragunov) in comparison with typical proteins. In addition, the proteins of plant residues are easily and quickly decomposed by soil microorganisms, their decomposition is accompanied by the resynthesis of the protein of the microbial plasma, which, in turn, is easily decomposed. Therefore, the hydrolysable part of the nitrogen of humic acid is represented, apparently, not by proteins, but by the products of their deep decomposition - amino acids, which are in the form of a fragile bond with the nucleus of humic acid. [...]
TOXINS are toxic substances produced by some microorganisms, plants and animals. By chemical nature - polypeptides and proteins. Sometimes the term T. also applies to non-protein poisons. The best studied microbial T., which are divided into exotoxins (excreted into the environment during growth) and endotoxins (released after the death of organisms). TOXIFICATION - an increase in toxicity as a result of the formation of new, more toxic substances during the decomposition (biological or physicochemical) of pesticides. Wed Pollutant, Harmful substance... TOXIC EFFECT OF A POLLUTANT - the harmful effect of a chemical on organisms (humans, animals, plants, fungi, microorganisms). With the combined toxic effect of several pollutants, a distinction is made between: the summation of harmful effects; over-summation, or potentiation; nihilation - the effect is less than that of summation; changes in the nature of the toxic effect (for example, the appearance of carcinogenic properties). TOXICITY - toxicity, the property of chemical compounds to have a harmful or even lethal effect on the body. [...]
Of considerable scientific and practical interest are water-insoluble cellulose grafted copolymers and biologically active proteins(enzymes, antigens). Graft copolymers of cellulose and enzymes can be used as specific catalysts, which can be easily removed from the reaction sphere at any time. The use of these copolymers makes it possible to solve a number of problems that cannot be solved using conventional water-soluble enzymes, for example, isolation of pure products of enzymatic decomposition of the substrate, isolation and subsequent study of intermediate products of enzymatic decomposition of the substrate, activation of the enzyme followed by complete removal of the activating substance, sorption, subsequent isolation and the study of enzyme inhibitors. Water-insoluble grafted copolymers of cellulose and antigens, which are called immunoadsorbents, are used for the adsorption of antibodies for the purpose of their quantitative determination, isolation in pure form for subsequent study and use. For the synthesis of water-insoluble graft copolymers of biologically active proteins, it is advisable to use cellulose rather than synthetic polymers, since the non-specific adsorption of protein on cellulosic materials is much lower than on synthetic polymers. [...]
The development of higher vegetation near water bodies is the reason for the ingress of dissolved organic products of their vital activity into the water and decay. As a result of the decomposition of macrophytes in water, proteins, carbohydrates, organic acids, tannins, as well as practically water-insoluble lignin, hemicellulose, fats, wax and resins can appear. [...]
In a living cell, the most varied and, moreover, multi-stage processes occur simultaneously: oxidation and reduction, synthesis and decay, transfer of methyl radicals, hydrolysis, etc. Some microbes have the ability to participate in a number of stages of decomposition of a substance. For example, they can use proteins and then carbohydrates, oxidize alcohols and acids, alcohols and then aldehydes, consume elemental nitrogen, and then bound nitrogen, etc. But there are also such microbes that are able to consume only certain certain carbohydrates and amino acids, not using others. [...]
Kelp fabrics consist of about 87% water and 13 ° / organic and mineral substances, the former being from 55 to 62% of the dry matter. Proteins that make up 5-7% of the dry residue in nutritional value correspond to soy protein and can be used as additives in animal feed. Kullny compares the thickets of the Gantt kelp with real underwater forests, which provide p) and shelter for a mass of marine organisms and fish. The same can be said about Japanese kelp thickets. These thickets will not lose the role of natural “protectors” of juveniles even during artificial production on ocean farms. [...]
The rate of chemical reactions in plant samples taken during the active growing season is much higher than in many analyzed objects (for example, grain, straw, seeds). Due to the work of enzymes, biochemical processes continue, as a result of which the decomposition of substances such as starch, proteins, organic acids and especially vitamins occurs. [...]
Other microbes that break down sugar, starch and even fiber, produce volatile acids, and nearby carbon, hydrogen and methane, unnecessary for the body, and thermal energy is only beneficial to the microorganism and is lost to the host's body. Finally, the third bacteria break down proteins, as well as enzymes, into small molecules of albumoses and peptones and further into amino acids and bases. But the activity of bacteria does not stop there, as it would be necessary for the host's organism, but leads further to the decomposition of these compounds into ammonia, fatty acids, alcohol and hydrocarbons that are not needed for the host. [...]
The main element of the aerobic biocenosis is the bacterial cell. A variety of multistage processes of transformation of organic substances take place in the cell. The biocenosis contains bacteria that are able to consume only certain carbohydrates or amino acids. Along with this, there are a large number of bacteria participating in several stages of the decomposition of organic matter. They can use proteins first, and then carbohydrates, oxidize alcohols, and then acids or alcohols and aldehydes, etc. Some types of microbes can lead the decomposition of organic matter to the end, for example, to the formation of carbon dioxide and water, others only to the formation of intermediate products ... For this reason, when treating wastewater, it is not individual cultures of microorganisms that give the necessary effect, but their natural complex, including more highly developed species [Rogovskaya Ts. I., 1967]. [...]
Vonros about the substances used in the process of respiration has long fascinated physiologists. Even in the works of I. II. Borodin showed that the intensity of the respiration process is directly proportional to the content of carbohydrates in plant tissues. This suggested that carbohydrates are the main substance consumed during respiration. In clarifying this issue great importance has a respiratory quotient definition. If carbohydrates are used in the process of respiration, then the process proceeds, according to the equation CeH 120b + 6O2 = 6CO2 + 6H2O, in this case the respiratory coefficient is equal to one - p = 1. However, if more oxidized compounds, for example, organic acids, undergo decomposition during respiration, oxygen uptake decreases, the respiratory coefficient becomes more than one. When more reduced compounds, such as fats or proteins, are oxidized during respiration, more oxygen is required and the respiration coefficient becomes less than one.
V metabolism of microorganisms nitrogen-containing substances undergo various transformations. By chance, superficial similarities, different types of food spoilage are often called rotting. However, decay is a process of deep decomposition of protein substances by microorganisms.
The ability to decompose protein substances to one degree or another is characteristic of many microorganisms. Some of them decompose proteins directly, others can only act on more or less simple breakdown products of a protein molecule, for example, peptides, amino acids, etc.
The decomposition products of proteins are used by microbes for the synthesis of substances in their body, as well as as an energy material.
Decomposition chemistry of protein substances. Rotting is a complex, multi-stage biochemical process, the nature and end result of which depends on the composition of the decomposed proteins, the conditions of the process and the types of microorganisms that cause it.
Protein substances cannot directly enter the cells of microorganisms, therefore, proteins can only be used by those microorganisms that have proteolytic enzymes - exoproteases secreted by cells into the environment.
The breakdown of proteins begins with their hydrolysis. The primary products of hydrolysis are peptones and peptides. They are broken down into amino acids, which are the end products of hydrolysis.
Various amino acids formed during the breakdown of proteins are used by microorganisms or undergo further changes by them, for example, deamination, resulting in the formation of ammonia "and various organic compounds. The process of deamination can occur in different ways. Distinguish between hydrolytic, oxidative and reductive deamination.
Hydrolytic deamination is accompanied by the formation of hydroxy acids and ammonia. If decarboxylation of the amino acid also occurs, then alcohol, ammonia and carbon dioxide are formed:
1 Due to the fact that ammonia is always present in the final products of protein breakdown, the process of decay is also called ammonification of protein substances.
During oxidative DeMining, keto acids and ammonia are formed:
During reductive deamination, carboxylic acids and ammonia are formed:
It can be seen from the above equations that among the decomposition products of amino acids, depending on the structure of their radical (R), various organic acids and alcohols are found. So, during the decomposition of fatty amino acids, formic, acetic, propionic, butyric and other acids, propyl, butyl, amyl and other alcohols can accumulate. In the decomposition of amino acids of the aromatic series, the intermediate products are characteristic decay products: phenol, cresol, skatole, indole - substances with a very unpleasant odor. The decomposition of amino acids containing sulfur produces hydrogen sulfide or its derivatives - mercaptans (for example, methyl mercaptan CH 3 SH). Mercaptans have a rotten egg smell that can be felt even at negligible concentrations.
The diamino acids formed during protein hydrolysis can be decarboxylated without the elimination of ammonia, resulting in diamines and carbon dioxide. For example, lysine is converted to cadaverine:
Likewise, ornithine is converted to putrescine.
Cadaverine, putrescine and other amines formed during putrefaction are often combined under the general name ptomaines (cadaveric toxins), some of which have poisonous properties.
Further conversion of nitrogenous and nitrogen-free organic compounds, resulting from the decomposition of various amino acids, depends on the environmental conditions and the composition of the microflora. Aerobic microorganisms oxidize these compounds so that they can be completely mineralized. In this case, the end products of putrefaction are ammonia, carbon dioxide, water, hydrogen sulfide, phosphoric acid salts. Under anaerobic conditions, there is no complete oxidation of intermediate decomposition products of amino acids. In this regard, in addition to ammonia and carbon dioxide, various organic acids, alcohols, amines and other organic compounds accumulate, including substances with poisonous properties, and substances that give a rotting material a disgusting smell.
Causative agents of putrefaction. Among the many microorganisms,
capable of decomposing proteins to one degree or another, microorganisms are of particular importance, which cause deep decomposition of proteins - actually decay. Such microorganisms are usually called putrefactive. Of these, bacteria are the most important. Putrefactive bacteria can be spore-forming and non-spore-forming, aerobic and anaerobic. Many of them are mesophiles, but there are cold-resistant and heat-resistant ones. Most are sensitive to the acidity of the environment.
The most common and active causative agents of putrefactive processes are the following.
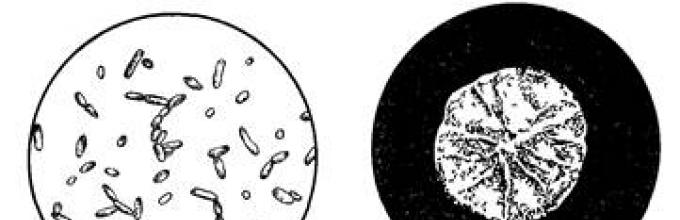
Hay and potato sticks 1 - aerobic, motile, gram-positive, spore-forming bacteria

Rice. 32. You. subtills:
a- sticks and oval spores; b - colony
(fig. 32). Their spores are highly heat-resistant. The optimum temperature for the development of these bacteria is 35–45 ° С, the maximum growth is at a temperature of about 50–55 ° С; at temperatures below 5 ° C, they do not multiply. In addition to the decomposition of proteins, such bacteria are able to decompose pectin substances, polysaccharides of plant tissues, and ferment carbohydrates. Hay and potato sticks are widespread in nature and are the causative agents of spoilage in many food products. They produce antibiotic substances that inhibit the growth of many pathogenic and saprophytic bacteria.
Bacteria of the genus Pseudomonas are aerobic motile rods, with a polar flagellum, which do not form spores, and are gram-negative (Fig. 33, a). Many "species are cold-resistant, the minimum temperature of their growth is from –2 to –5 ° C, the optimum is about 20 ° C. Many pseudomonas, in addition to proteolytic, have lipolytic activity; they are able to ferment carbohydrates with the formation of acids, secrete mucus. Development
1 In accordance with the International Code of Nomenclature for Bacteria, hay and potato sticks are considered synonyms of the same species - Bacillus subtilis.
and the biochemical activity of these bacteria is significantly inhibited at pH below 5.5 and 5–6% NaCl concentration in the medium. Pseudomonas are widespread in nature, they are antagonists of a number of bacteria and molds, as they form antibiotic substances. Some Psudomo-nas species are the causative agents of diseases (bacterioses) of cultivated plants, fruits and vegetables.
Proteus (Proteus vulgaris) - small gram-negative sporeless sticks with pronounced putrefactive properties. Protein substrates acquire a strong putrid odor during the development of Proteus in them. Depending on the conditions

Rice. 33.
a - Pseudomonas; b - Proteus vulgaris
In life, these bacteria are able to noticeably change their shape and size (Fig. 33, b).
Proteus - facultative anaerobe; ferments carbohydrates to form acids and gases. It develops well both at 25 ° C and 37 ° C, ceasing to multiply only at a temperature of about 5 ° C, but it can persist in frozen foods.
A characteristic feature of Proteus is its very energetic mobility. This property forms the basis of the method for detecting proteus in food and separating it from accompanying bacteria. Some types of proteus emit substances toxic to humans (see p. 159).
Clostridium putrificum (Fig. 34, a)- anaerobic mobile, spore-forming stick. Its relatively large spores are located closer to the end of the cell, which at the same time acquires a resemblance to a drumstick. The spores are quite thermally stable. This bacteria does not ferment carbohydrates. Proteins decompose with the formation of a large amount of gases (NH 3, H2S). The optimum temperature for development is 37–43 ° С, the minimum is 5 ° С.
Clostridium sporogertes (Fig. 34, b)- anaerobic mobile spore-bearing bacillus. Spores are thermally stable, in the cell they are located closer to its end. The very rapid (during the first days of growth) spore formation is characteristic. This bacterium ferments carbohydrates with the formation of acids and gases, has a lipolytic ability. When proteins are decomposed, hydrogen sulfide is liberated abundantly. The optimum temperature for development is 35–40 ° С, the minimum is about 5 ° С.
Both types of Clostridia are known to cause spoilage in canned food (meat, fish, etc.).

Rice. 34.
a - Clostridium putrificum; b - Clostridium sporogenes
The practical importance of decay processes. Putrefactive microorganisms often cause great damage national economy, causing spoilage of the most valuable and protein-rich food products, such as meat and meat products, fish and fish products, eggs, milk, etc. But these microorganisms play a large positive role in the cycle of substances in nature, mineralizing protein substances that enter the soil and water.
Putrid processes. The concept of aerobic and anaerobic decay. Pathogens. The role of putrefactive processes in nature, in the food industry
Rotting is a process of deep decomposition of protein substances. One of the end products of the decomposition of protein substances is ammonia; therefore, the decay process is called ammonification.
Proteins are high molecular weight compounds, therefore, at first they undergo extracellular cleavage by proteolytic enzymes of microorganisms, which are exoenzymes.
The breakdown of proteins occurs in steps:
proteins> peptones> polypeptides> amino acids
The formed amino acids diffuse into cells and can be used both in constructive and energy metabolism.
The breakdown of amino acids begins by deamination and decarboxylation. During the deamination of amino acids, the amino group is cleaved with the formation of ammonia, organic acids (butyric, acetic, propionic, hydroxy and keto acids) and high molecular weight alcohols.
In the future, the formation of final products depends on the conditions of the process and on the type of microorganism - the causative agent of putrefaction.
Aerobic decay. It flows in the presence of atmospheric oxygen. The end products of aerobic decay are, in addition to ammonia, carbon dioxide, hydrogen sulfide and mercaptans (with the smell of rotten eggs). Hydrogen sulfide and mercaptans are formed during the decomposition of sulfur-containing amino acids (cystine, cysteine, methionine).
Anaerobic decay. It proceeds under anaerobic conditions. The end products of anaerobic putrefaction are the products of decarboxylation of amino acids (removal of the carboxyl group) with the formation of foul-smelling substances: indole, akatol, phenol, cresol, diamines (their derivatives are cadaveric poisons and can cause poisoning).
Causative agents of putrefactive processes
The causative agents of aerobic putrefaction are spore-forming bacteria of the genus Bacillus: Bacillus mycoides (pear-shaped bacillus); Bacillus megaterium (cabbage bacillus); Bacillus mesentericus (potato stick); Bacillus subtilis (hay bacillus), as well as non-spore-forming sticks: Serrate marcencens (wonderful stick); Proteus vulgaris (Proteus stick); Escherichia coli (Escherichia coli) and other microorganisms.
The causative agents of anaerobic putrefaction are anaerobic spore rods of the genus Clostridium (proteolytic clostridia): Clostridium sporogenes, Clostridium subterminalis, Clostridium perfringens, Clostridium botulinum.
The practical significance of putrefactive processes
Putrefactive microorganisms often cause great damage to the national economy, causing spoilage of protein-rich food products: meat and meat products, eggs, milk, fish and fish products, etc.
In nature (in water, soil) putrefactive bacteria actively decompose dead animal and plant tissues, mineralize protein substances and thus play an important role in the cycle of carbon and nitrogen.
Decomposition of cellulose and pectin substances by microorganisms
The decomposition of pectin substances is close to butyric acid fermentation. It proceeds under anaerobic conditions. Under the influence of pectolytic enzymes of microorganisms, prototopectin is converted into soluble pectin, which decomposes with the formation of galacturonic acids, carbohydrates (xylose, galactose, arabinose), methyl alcohol and other substances. Further, the sugars are fermented by bacteria of the genus Clostridium with the formation of butyric and acetic acids, carbon dioxide and hydrogen.
All these processes lead to mineralization (decay) of the affected objects (fruits, vegetables) and to other types of damage.
Fermentation of cellulose consists in its decomposition under anaerobic conditions with the formation of butyric, acetic acids, carbon dioxide, ethyl alcohol, hydrogen. This process is carried out by spore-forming mesophilic and thermophilic cellulose bacteria belonging to the genus Clostridium.
In aerobic breakdown of fiber, the end products are carbon dioxide and water. Aerobic microorganisms that oxidize fiber include mesophilic aerobic bacteria of the genera Cytophaga, Anginococcus. Cellvibrio, Pseudomonas, actinomycetes of the genus Streptomyces and microscopic fungi (genera Penicillium, Alternaria, Fusarium, etc.).
In nature, pectin-decomposing and cellulose bacteria play an important role in the decomposition of plant residues and, therefore, in the carbon cycle.
Ammonification chemistry.
Rotting corpse (putrification of a corpse,
p
utrefactio
mortis
)
- decomposition of the organic matter of the corpse under the action of the enzyme systems of microorganisms with the formation of final inorganic products.
Typical decay products are water, carbon dioxide, ammonia, hydrogen sulfide, volatile fatty acids (formic, acetic, butyric, valerian and nylon, as well as isomers of the last three acids), phenol, cresol, indole, skatole, amines, trimethylamine, aldehydes, alcohols , purine bases, etc. Some of these substances arise in the process of decay, others are contained in the corpse, but during decay their amount increases many times. A fairly large number of various aerobic, facultative anaerobic and anaerobic spore-forming and spore-forming bacteria are involved in decay.
At a storage temperature of about 0 ° C, decay is mainly due to the vital activity of psychrophilic bacteria, most often of the genus Pseudomonas. At elevated storage temperatures, protein decay is mainly caused by mesophilic putrefactive microorganisms: non-spore-forming bacteria - the common Proteus stick (Proteus vulgaris), the miraculous stick (Serratia marcescens), the hay stick (Bac. Subtilis), the potato stick (Bach. Mesentericus), the mushroom stick (Bach . mycoides) and other aerobic bacilli; anaerobic clostridia - sporogenes stick (Cl. sporogenes), putrificus stick (Cl. putrificus) and perfringens stick (Cl. perfringens). Molds can also participate in the decay processes.
In most cases, the species composition of the bacterial flora that develops during rotting in corpses depends on the nature of the bacteria in the gastrointestinal tract of the deceased.
Putrification of a corpse is a sequential multistage process, each step of which proceeds with the formation of a certain number of decomposition products, which undergo further sequential transformations.
The staging of the course of decay processes is due to the unequal enzymatic activity of the putrefactive microflora in relation to various substances. Proteins that are in a dissolved state, such as blood proteins and cerebrospinal fluid proteins, are more susceptible to the action of microorganisms. The transformation of the decay products of proteins occurs through intermediate substances with the formation of final, foul-smelling decay products. In the putrefactive decay of a corpse, various microorganisms can both simultaneously and sequentially participate: primarily those that are capable of destroying protein molecule and then microbes assimilating protein breakdown products.
In total, as a result of the putrification of corpses, about 1300 different compounds can be formed in stages, whose chemical composition depends on the decomposition time of the cadaveric material, temperature, the presence of moisture, air access, bacterial flora, the composition of organs and tissues undergoing decomposition, as well as on a number of others. factors.
One of the initial products of putrefactive decomposition of proteins are peptones (mixtures of peptides), which can cause poisoning when administered parenterally. Peptides decompose to form mercaptants (thioalcohols and thiophenols) and amino acids. The free amino acids formed during the hydrolysis of peptones undergo deamination, oxidative or reductive decarboxylation. During the deamination of amino acids, volatile fatty acids (caproic, isocaproic, etc.) are formed, and during decarboxylation, various toxic organic bases - amines. Amino acids containing sulfur decompose with the release of methyl mercaptan, hydrogen sulfide and other sulfur compounds.
The greatest activity of influence on proteins is exerted by aerobes - B. proteus, B. pyocyaneum, B. mesentericus, B. subtilis, streptococci and staphylococci; anaerobes - Cl. putrificus, Cl. histolyticus, Cl. perfringens, Cl. Sporogenes, B. bifidus, acidofilus, B. butyricus ... Aerobes break down amino acids - B. faecalis alcaligenes, B. lactis aerogenes, B. aminoliticus, E. coli, etc.
When lipoproteins decay from them, first of all, the lipid part is split off. An integral part of Lecithin, found in muscles, as well as in the brain and spinal cord, is choline, which, during the process of decay, turns into trimethylamine, dimethylamine and methylamine. Trimethylamine upon oxidation forms trimethylamine oxide, which has a fishy odor. In addition, the poisonous substance neurin can be formed from choline during rotting of the corpse.
During the putrefactive decomposition of carbohydrates, organic acids, products of their decarboxylation, aldehydes, ketones, lactones, and carbon monoxide are formed.
When decaying, nucleoproteins decompose into protein and nucleic acid, which then breaks down into its constituent parts, resulting in the formation of hypoxanthine and xanthine - the decomposition products of nucleoproteins.
Biogenic diamines formed as a result of partial decomposition of proteins and decarboxylation of their amino acids and possessing a toxic effect are collectively called "cadaveric poison". Organic bases (ethylenediamine, cadaverine, putrescine, skatole, indole, ethylenediamine, etc.), formed during protein decay, are also called ptomaines (from the Greek - Πτώμα, meaning a dead body, corpse).
The main toxic substances of these are putrescine and cadaverine, as well as spermidine and spermine. Putrescine, 1,4 - tetramethylenediamine, H 2 N (CH 2) 4 NH 2; belongs to the group of biogenic amines. Crystalline substance with an extremely unpleasant odor, melting point 27-28 ° C. It was first discovered in the products of putrefactive decomposition of proteins. Formed by decarboxylation of the amino acid ornithine by bacteria. In body tissues, putrescine is the initial compound for the synthesis of two physiologically active polyamines - spermidine and spermine. These substances, along with putrescine, cadaverine and other diamines, are part of the ribosomes, participating in the maintenance of their structure.
Cadaverine (from Lat.cadaver - corpse), α, ε-pentamethylenediamine - chemical compound having the formula NH 2 (CH 2) 5 NH 2. It got its name because of its very strong cadaveric smell. It is a colorless liquid with a density of 0.870 g / cm3 and a boiling point of 178-179 ° C. Cadaverine is readily soluble in water and alcohol, gives well crystallizing salts. Freezes at +9 ° C. Contained in the products of putrefactive decomposition of proteins; formed from lysine during its enzymatic decarboxylation. Found in plants. Artificially, cadaverine can be obtained from trimethylene cyanide.
Spermine is a chemical in the aliphatic polyamine class. Participates in cellular metabolism, is found in all eukaryotic cells, in living organisms it is formed from spermidine. Spermine was first isolated in 1678 from human sperm by Anthony van Leeuwenhoek as a crystalline salt (phosphate). The name "spermine" was first used by German chemists Ladenburg and Abel in 1888. At present, spermine is found in various tissues of a large number of organisms, and is a growth factor in some bacteria. At physiological pH, it exists as a polycation.
It should be noted that the toxicity of chemically pure ptomains is low compared to the action of the cadaveric material itself. In experiments on rats, the toxic dose of cadaverine is 2000 mg / kg, putrescine - 2000 mg / kg, spermidine and spermine - 600 mg / kg.
Therefore, the poisonous properties of the cadaveric material are explained by the action of certain impurities (bacterial toxins and a number of synthesis products formed in the cadaveric material under the influence of bacterial enzymes) contained along with polyamines in the putrefactive biological material.
Decay can occur both when oxygen is available to the tissues of the corpse (aerobic decay) and in its absence (anaerobic decay). As a rule, aerobic and anaerobic types of putrefaction develop simultaneously, we can only talk about the predominance of one or another process.
Under aerobic conditions, protein breakdown proceeds mainly with the participation of aerobic microorganisms (B. proteus vulgaris, B. subtilis, B. mesentericus, B. pyocyaneum, B. coli, Sarcina flava, Streptococcus pyogenes, etc.) and the formation of many intermediate and final decay products. Aerobic decay proceeds relatively quickly and is not accompanied by the release of a large amount of liquid and gases with a specific fetid odor. Rotting under the influence of aerobic microorganisms with good oxygen access occurs with more complete oxidation. At the same time, aerobes greedily absorb oxygen and thereby contribute to the development of anaerobes.
Under anaerobic conditions, fewer putrefaction products are formed, but they are more toxic. Anaerobic microorganisms (B. putrificus, B. perfringens and others) cause a relatively slower decay, in which the oxidation and decomposition of biological compounds is not complete enough, which is accompanied by the release of a large amount of liquid and gases with a fetid odor.
In addition to biochemical stages, the staging of rotting of a corpse is also characterized by morphological, relatively constant, developmental periods.
Under standard conditions, decay begins within 3-4 hours after death, and at the initial stage it proceeds imperceptibly. The bacterial putrefactive flora found in the large intestine is activated, which leads to the formation of a large number of gases, and their accumulation in the intestines and abdomen. Bloating of the intestine, an increase in the volume of the abdomen and some tension of the anterior abdominal wall by palpation can be noted as early as 6-12 hours after the death of a person.
The resulting putrefactive gases, which include hydrogen sulfide, penetrate the intestinal walls and begin to spread through the blood vessels. Combining with blood hemoglobin and muscle myoglobin, hydrogen sulfide forms compounds - sulfhemoglobin and sulfmyoglobin, which impart a dirty green color to internal organs and skin.
The first external signs of decay become noticeable on the anterior abdominal wall by the end of 2 - the beginning of the third day after death. Dirty green skin coloration appears, appearing first in the right iliac region, and then in the left. This is due to the fact that the large intestine is directly adjacent to the anterior abdominal wall in the iliac regions. In summer or in warm conditions, the dirty green color of the skin in the iliac regions may appear a day earlier.
 Rice. "Corpse Greens". Dirty green coloration of the skin in the iliac regions
Rice. "Corpse Greens". Dirty green coloration of the skin in the iliac regions
Since blood proteins are easily rotted, putrification quickly spreads through the blood vessels to other areas of the body. Rotting blood further enhances its hemolysis and increases the amount of sulfhemoglobin, which leads to the appearance of a branched, dirty-brown or dirty-green venous pattern on the skin - a subcutaneous putrefactive venous network. Distinctly distinguishable signs of a putrid venous network are noted as early as 3-4 days after death.
 Rice. Putrid venous network
Rice. Putrid venous network
On the 4th - 5th day, the entire anterior skin of the abdominal wall and genitals acquires a uniform dirty green tint, and cadaverous greenery develops.
By the end of the 1st - the beginning of the 2nd week, the dirty green staining covers a significant part of the surface of the corpse.
At the same time, as a result of the binding of hydrogen sulfide (H 2 S) formed during decay with iron released as a result of hemolysis of erythrocytes and the breakdown of hemoglobin, iron sulfide (FeS) is formed, which gives a black color to soft tissues and parenchyma of internal organs.
Black staining of corpse tissues (cadaveric pseudomelanosis, pseud ome l anosis) occurs unevenly and is most clearly seen in those places in which the greatest accumulation of blood is noted - in the area of cadaveric spots and hypostases.
The noted order of development of putrefactive manifestations during external examination is observed in most cases, however, there may be exceptions. For example, upon death from mechanical asphyxia, cadaverous greenery initially appears not in the iliac regions, but on the head and chest. This is due to the fact that the stagnation of blood in the upper part of the body formed during asphyxia contributes to the development of putrefaction in these areas of the body.
In the process of decay, a variety of coccal and rod flora begins to develop on the surface of the corpse, as a result of which its skin becomes slick. The corpse is covered with shiny mucus, or semi-dry grease, similar to yellow-red or brown fat.
In cases where a corpse is found in conditions of low temperatures and low humidity, mold growth can be observed on the surface of the corpse. Unlike putrefactive microorganisms, molds can develop in an acidic environment (pH 5.0-6.0), at relatively low air humidity (75%) and low temperatures. Some types of mold grow at a temperature of 1-2 ° C, while others grow at minus 8 ° C or even lower.
Molds develop rather slowly, therefore, mold of a corpse mainly occurs when it is kept for a long time in the conditions noted above or in a refrigerator. Mold fungi are aerobic microorganisms and, as a rule, develop most actively in those areas of the corpse, on the surface of which air movement is most intense, as well as in more humid areas (inguinal and axillary folds, etc.).
Depending on the species, mold can grow in the form of round, velvety colonies of white, dark gray-brown or greenish-bluish, as well as black, located on the surface of the skin or penetrating into the thickness of soft tissues to a depth of 1.0 cm. corpse is relatively rare, since psychrophilic aerobic bacteria actively multiplying on the surface of the corpse usually inhibit the growth of mold fungi.
If the corpse has been in sea water, or near fresh seafood, a faint glow of the surface of the corpse may be observed. This phenomenon is quite rare and is due to the multiplication of photogenic (luminous) bacteria on the body surface, which have the ability to glow - phosphorescence. The luminescence is due to the presence in the cells of luminous bacteria of a photogenic substance (luciferin), which is oxidized by oxygen with the participation of the luciferase enzyme.
Photogenic bacteria are obligate aerobes and are psychrophilic; they multiply well, but do not cause changes in the smell, consistency, and other parameters of the corpse. The group of photobacteria includes various non-spore-forming gram-negative and gram-positive rods, cocci and vibrios. A typical representative of photogenic bacteria is photobacterium phosphoreum (Photobact. Phosphoreum) - a mobile coccoid bacillus.
With the development of putrification, putrefactive gases are formed not only in the intestines, but also in the soft tissues and internal organs of the corpse.
On the 3-4th day of the development of putrefaction on palpation of the skin and muscles, crepitus is clearly felt, there is an increase in the accumulation of putrefactive gases in the subcutaneous fat and in other tissues - cadaveric emphysema develops. First of all, putrefactive gases appear in the fatty tissue, then in the muscles ..
By the end of the second week, cadaveric gigantism develops - the penetration of gases into soft tissues leads to an increase in the volume of the corpse. In a corpse, parts of the body sharply increase in size: the abdomen, chest, limbs, neck, in men, the scrotum and penis, in women, the mammary glands.
With putrefactive changes in the subcutaneous fat, the facial features change dramatically: it becomes dark green or purple, swollen, the eyelids swell, the eyeballs protrude from the orbits, the lips increase in size and turn outward, the tongue is enlarged in size from behind the mouth. A dirty-red, bloody fluid is discharged from the mouth and nose.
 Rice. "Corpse gigantism". An increase in the size of the corpse due to the development of putrefactive emphysema
Rice. "Corpse gigantism". An increase in the size of the corpse due to the development of putrefactive emphysema
The pressure of putrefactive gases in the abdominal cavity can be quite significant and reach 1-2 atm., Which leads to the development "Posthumous birth" (grave birth, partus post mortem ) - squeezing the fetus through the birth canal from the uterus of the corpse of a pregnant woman with gases formed in the abdominal cavity during rotting of the corpse. As a result of the accumulation of putrefactive gases in the abdominal cavity, an eversion outward from the genital tract of the uterus and the release of gastric contents from the oral cavity ( "Posthumous vomiting" ).
Further increased pressure of putrefactive gases in the abdominal cavity and the gradually decreasing strength of the tissues of the anterior abdominal wall as decay develops lead to its rupture and eventration of the contents of the abdominal cavity.
Due to the extravasation of fluid, by about the end of the 1st week, putrefactive blisters are formed under the epidermis, containing a reddish-brown fetid bloody liquid. Putrefactive blisters easily rupture, the epidermis is rejected, exposing the moist, reddish surface of the skin itself. Such manifestations of decay mimic skin burns. Putrid skin changes cause hair loss or slight rejection.
On days 6-10, the epidermis completely exfoliates and, with a slight mechanical effect, can be easily removed along with nails and hair.
 Rice. Putrid rejection of the skin and nail plates
Rice. Putrid rejection of the skin and nail plates
Further, through the damaged areas of the skin, putrefactive gases leave the corpse. The size of the corpse and its parts is reduced. Softening of nails, skin and their further separation is observed. The skin becomes yellowish, easily torn, covered with papillae, which are outwardly similar to grains of sand and are composed of phosphate lime.
Two weeks later, a reddish putrefactive liquid (ichor) begins to stand out from the natural openings of the corpse, which should not be mistaken for traces of intravital bleeding.
In the future, the skin of the corpse becomes thinner, becomes thin, dirty yellow or orange with mold.
In the third week, the decomposition of the corpse intensifies. The tissues are becoming more and more slimy, easily torn. The soft parts of the face fall off. The muscles are soft, the fiber begins to dry out (drying begins from the front and sides). The muscles of the eye sockets are saponified or turn green.
As the putrefactive decay progresses, the formation of putrefactive gases stops, cadaveric emphysema disappears, and the volume of the corpse decreases. The processes of putrification soften, disorganize the tissues - the so-called putrefactive melting of the corpse takes place.
The subcutaneous tissue is partially saponified, as a result of drying and collapse of cells, previously stretched by putrefactive gases, on the cut has a "borehole" appearance. Cartilage and ligaments turn yellow, become flabby and easily expandable. Muscles become flabby and sticky, easily torn with slight stretching, transforming as putrification into a structureless brown-black mass or layers of gray-yellow color with indistinguishable muscle fibers. Bones, especially in those places where they are covered with a small amount of soft tissue, are exposed, the ribs are easily separated from the cartilage.
Rotting of internal organs proceeds unevenly. Starting in the intestines and abdomen, it primarily captures the nearby abdominal organs (liver, pancreas and spleen). The macroscopic structure of internal organs is completely lost as it decays. Internal organs decrease in volume, crepitus on palpation, easily flattened, torn. Putrefactive gases destroy the structure of the parenchyma, organs in the cut acquire a "foamy", "porous" appearance, the removed pieces of organs float on the surface of the water due to putrefactive gases.
The peritoneum becomes licky, turns green. The mucous membranes of the stomach and intestines become brownish-purple in color, sometimes with small discolored areas. In some cases, there is a perforation of the fundus of the stomach with the outflow of gastric contents into the abdominal cavity or into the left pleural cavity. However, this phenomenon is not a consequence of putrefaction, but occurs as a result of cadaveric autolysis. The putrefactive process in the lungs is accompanied by the appearance of gas, bubbles in the vessels, in the interstitial tissue and under the pleura.
The lungs are dark red in color and of a loose consistency, filled with a sanguine liquid. Gradually, as it decays, most of the ichor accumulates in the pleural cavities.
When decaying, lymph nodes are soft, can be of different colors: brown-red, greenish, dark brown, black.
The heart is flabby, the walls of the chambers are thinned, the myocardium is dirty red on the cut. On the surface of the endocardium and pericardium, small white granules of calcareous deposits are noted. The pericardium is macerated, the pericardial fluid is turbid, with a flocculent sediment. With cadaveric hemolysis with imbibition of the blood pigment of the tissues, the pericardial fluid from the admixture of hemoglobin can turn brownish-red.
The liver in the process of decay softens, grows dull, emits a strong ammonia odor. First, the lower surface of the liver, and then the front and back, become black. On the surface of the liver, "sandy" papillae made of phosphate lime are visible. In the thickness of the parenchyma, multiple bubbles are formed, filled with putrefactive gases, which gives the liver tissue a honeycomb, foamy appearance on the cut. The outpouring and release of bile outside the gallbladder during decay leads to the appearance of a yellow-green coloration of the lower edge of the liver and nearby tissues and organs.
The pancreas early undergoes rotting, during which it becomes flabby, with an indistinguishable structure, in the form of a gray mass.
The spleen is reduced in size, flabby, the pulp of the spleen turns into a red-black or greenish-black, semi-liquid, sometimes frothy, fetid mass from the presence of gases.
Due to the topographic proximity of the spleen to the large intestine, hydrogen sulfide easily penetrates into it from the intestine already in the first days after death, which, when combined with the iron of hemoglobin, forms iron sulfide, which first stains the part of the spleen adjacent to the intestine, and later the entire organ in greenish-black or bluish -black color.
The brain completely loses its anatomical structure, the border of gray and white substances becomes indistinguishable, its consistency at the beginning acquires a mushy, and subsequently semi-liquid state. Putrefactive decay of the bone marrow occurs later than in other tissues. This is due to the late penetration of microorganisms into the bone marrow of the corpse.
The most resistant to decay are blood vessels, organ stroma, non-pregnant uterus, prostate and cartilage.
The complete putrefactive decay of the soft tissues of a corpse under conditions favorable for the development of putrification processes can occur already after 3-4 weeks.
Histological examination in the presence of putrefactive changes is of relative importance. With moderate rotting in the lungs, "stamped" alveoli are determined, the outlines of the bronchi, carbon pigment are visible, in the lung parenchyma, Gram-positive rods can be found, forming figures in the form of threads and brushes.
As a result of putrefactive transformation, liver tissue quickly loses its histological structure, due to diffusion into the parenchyma of bile and blood, a lot of greenish-brown pigment is found in it. The follicles of the spleen during the processes of cadaveric softening and decay are preserved better than the elements of the pulp. Even with the complete putrefactive decay of the pulp cells, the nuclei of the lymphoid elements of the follicles still give color. When the spleen is fixed in formalin, formalin pigment easily falls out, settling on the pulp cells, which leads to pigmentation of the tissue of the spleen, stroma and erythrocytes, which complicates microscopic examination.
The kidneys, in comparison with the liver, are more resistant to decay; they are histologically verified by the outlines of the glomeruli and blood vessels.
Microscopic examination of the putrefactive lymph nodes reveals the disappearance of the nuclear color of the lymphoid elements and their decay. Stromal elements remain in the lymph nodes for a little longer.
Decay of muscle tissue is accompanied by a change in the structure of muscle fibers: their transverse striation is smoothed out and disappears, the nuclei are poorly stained, fine-grained decay, divergence and complete destruction of muscle fibers are observed.
With slightly pronounced rotting histological examination allows you to identify some pathological changes, and with the complete destruction of cellular elements, differentiate organs according to the structure of the organ stroma and blood vessels. So, for example, it is possible to establish sclerotic changes and calcification of large arterial vessels even several months after death, sometimes fragments of powder grains can be found in the putrefactively transformed parenchyma. However, in most cases, with pronounced putrification, microscopic examination of the material can add practically nothing to the data of macroscopic examination.
When conducting a forensic chemical study of cadaveric material in a state of putrefactive transformation and interpreting its results, it should be borne in mind that a number of substances formed in the tissues of corpses during decay can give the same reactions as some poisons of organic origin.
This circumstance can significantly complicate the process of detecting and quantifying poisons in chemical-toxicological analysis, and also cause erroneous conclusions about the presence of poisons in the organs of corpses.
Thus, the assessment of the alcohol content in the putrefactive biological material requires great care.
It should be borne in mind that as a result of the vital activity of a number of bacteria participating in the putrification of corpses, amino acids and fats are oxidized to form alcohols, the mixture of which contains methyl, ethyl and higher alcohols. Under the influence of E. coli enzymes, different amounts of propyl, butyl and methyl alcohols are formed from glucose. Amyl alcohol is formed from leucine, and isobutyl alcohol is formed from valine.
The quantitative content of posthumous alcohols, as a rule, is insignificant and ranges from 0.5 ppm, but occasionally it can reach 1.0 ppm or more.
The exception is those cases when yeast flora is present in the cadaveric material. In this case, the amount of posthumously formed alcohols, in particular ethyl alcohol, can reach toxicologically significant levels.
In the process of putrefactive decomposition of corpses chemical changes some of the poisonous substances that caused the poisoning are also exposed.
The speed and intensity of the transformation of toxic substances in the putrefied corpse depends on a number of general factors affecting the decay process, as well as on the chemical nature of the poisons, the palette of cadaveric bacterial flora, access to air, moisture, decay time, and other conditions.
Toxins of organic origin in rotting corpses undergo oxidation, reduction, deamination, desulfurization and other transformations, which leads to their relatively rapid decomposition.
Esters decompose most quickly, within a few days or weeks after death, however, some toxic substances (atropine, cocaine, etc.) belonging to the noted class of compounds can be found in corpses several months or years after death.
Inorganic poisonous substances in cadaveric material persist longer, undergoing recovery reactions during rotting corpses. The metal ions in inorganic poisons, which have a higher valence, are reduced to ions with a lower valence. Compounds of arsenic, phosphorus, sulfur and other non-metals can be reduced to form volatile compounds of these elements with hydrogen.
Arsenic and thallium compounds can persist in corpses for about 8-9 years, barium and antimony compounds for about 5 years, mercury compounds remain in corpses for several months. After that, inorganic poisons penetrate the soil and cannot always be found in the remains of rotting or decayed corpses.
Despite the fact that the general biochemical nature of putrefaction is quite constant, individual characteristics of the putrification process are quite labile and depend on a number of factors:
Environmental conditions;
the location of the corpse (outdoors, in water, in the ground);
anthropometric characteristics of the corpse;
the nature of the clothes on the corpse;
the age of the deceased;
the presence of damage;
causes of death;
drugs taken before death;
composition of microflora, etc.
Temperature and humidity environment directly affect the rate of putrefactive transformation of a corpse. The most optimal conditions for the life of putrefactive microorganisms arise at a temperature of + 30 -37 ° C, high humidity and access to oxygen in the air. Rotting almost completely stops at a body temperature of the deceased about 0 ° C and above + 55 ° C and sharply slows down in the range from 0 ° C to + 10 ° C, due to unfavorable temperature conditions for the reproduction of putrefactive microorganisms.
Under appropriate temperature and humidity conditions, the development of putrefactive microorganisms in a corpse is possible extremely quickly, which leads to the fact that putrefaction in time can outpace the process of autolysis.
If, after death, the process of tissue drying (mummification) develops, then the decay gradually slows down, and then stops altogether.
In conditions of high humidity (for example, when a corpse is in water), the course of decay slows down sharply, which is explained by a lower oxygen concentration and a lower temperature. In dry, sandy, well-ventilated soil, rotting develops faster than in dense clay, poorly ventilated soil. Corpses buried in coffins and in clothes are subject to rotting more slowly than simply buried in the ground and without clothes.
Cases of almost complete absence of putrefactive changes are described after a long period of time after burial (up to 53 years) when the corpse is in metal coffins (zinc, lead). Rotting of a corpse in the ground proceeds eight times slower than in air.
The development of decay is greatly influenced by the individual characteristics of the corpse.
The corpses of children undergo putrefactive decomposition faster than the corpses of adults, while the corpses of newborns and stillborns rot more slowly due to the absence of putrefactive flora.
In the corpses of obese people, decay develops faster than in the corpses of thin or emaciated ones.
Accelerated decay is observed when the onset of death was accompanied by severe agony, death, in cases of death from infectious diseases, with septic complications, with extensive damage to the skin, with overheating (the so-called heat or sunstroke), as well as with some intoxications.
A slowdown in putrefaction is noted with death from massive blood loss, with the intravital intake of antibiotics, sulfa and other antimicrobial drugs.
With dismemberment, which is always accompanied by a sharp exsanguination of body parts, a slowdown in the processes of decay leads to a longer preservation of parts of the dismembered corpse.
The rotting of a corpse in the conditions of its stay in water has its own distinctive features... Rotting in a pond with running water is slower than in standing water... When a corpse hits the bottom of a reservoir with great depth, where the water temperature is. + 4 ° С and high pressure, the decay process may not develop for many months.
When a corpse is found at a depth of the reservoir, its rotting proceeds relatively slowly and evenly. After a two-week stay in water, the corpse begins to lose hair, but hydro-depilation is completely completed by the end of the month.
Putrefactive gases accumulating in the tissues and cavities of the corpse increase its buoyancy, due to which the corpse floats to the surface of the water. The lifting force of putrefactive gases is so great that a corpse weighing 60-70 kg can float up together with a load weighing about 30 kg. At a water temperature of 23-25 ° C, the emergence of a corpse to the surface of the water occurs on the 3rd day, at a water temperature of 17-19 ° C, the emergence of a corpse occurs on the 7-12th day, in colder water the emergence of a corpse occurs after 2-3 weeks.
After the corpse ascends to the surface of the water, the decay process increases abruptly and proceeds unevenly. The soft tissues of the face swell and turn green, while other parts of the body may be little affected by decay. In the future, the whole body swells up and the corpse is disfigured, the belly swells up sharply, the corpse takes on the appearance of a "giant", which can lead to errors in identifying the body of an unknown person. Especially the scrotum increases in volume, the tissues of which, under the influence of gases, can rupture.
In warm weather, corpses taken out of water in the air decompose very quickly. Within a few hours, signs of decay appear - a dirty green color of the skin, a putrid venous network. Due to the fact that the development of putrification processes is influenced by a large number of factors that are not always possible to take into account in aggregate, the forensic medical determination of the prescription of death by the nature and severity of putrefactive changes can only be carried out approximately.
Putrefactive transformations of a corpse make very tangible changes in the structure of tissues and organs, destroying many pathological changes that were present during life, however, a forensic medical examination of corpses should be carried out regardless of the degree of decay. Even with pronounced putrefactive changes in the course of a forensic medical examination, it is possible to detect damage and other signs that will make it possible to establish the cause of death and resolve other issues that arise before an expert.
Forensic medical expert, associate professor of the Department of Forensic Medicine at the Russian National Research Medical University named after V.I. N.I. Pirogov of the Ministry of Health of Russia, Ph.D. Sci., Associate Professor Tumanov E.V. T Umanov E.V., Kildyushov E.M., Sokolova Z.Yu. Forensic thanatology - M .: YurInfoZdrav, 2011 .-- 172 p.